Ester to Alcohol
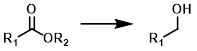
Common Conditions:
LiAlH4
Lithium Aluminum Hydride (LiAlH4) is often the reagent of choice for reducing esters to alcohols because it is a very powerful reducing agent. One drawback is its lack of selectivity.[1]
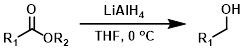
DIBAL-H
Diisobutylaluminum Hydride (DIBAL-H) is a powerful reducing agent which offers more selectivity than LiAlH4. Another advantage of DIBAL-H over LiAlH4 is its solubility not only in ethers, but in a wide range of hydrocarbon solvents (ex. DCM, toluene, and hexane).[2]
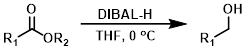
LiBH4
Lithium Borohydride (LiBH4) is less reactive than LiAlH4 but much more reactive than NaBH4. LiBH4, unlike NaBH4, has good solubility in THF.[1]
BH3-SMe2
In refluxing THF, borane-dimethyl sulfide (BH3-SMe2) generally reduces aliphatic esters quickly (0.5 h), while aromatic esters require more time (4-16 h).[3]
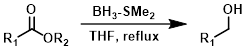
NaBH4
Sodium Borohydride (NaBH4) tends to reduce esters slowly but is sometimes a useful alternative to the stronger reducing agents. The most common solvents are MeOH and EtOH.[1]
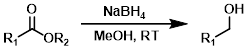
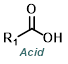
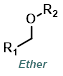
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
 |
||||
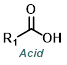 |
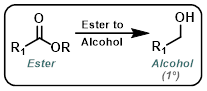 |
 |
||
 |
||||
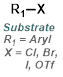 |
 |
|||
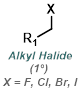 |
||||
References:
1) www.acros.com, Aluminum Hydrides and Borohydrides (Link)
2) www.akzonobel.com, Diisobutylaluminum hydride (DIBAL-H) and Other Isobutyl Aluminum Alkyls (DIBAL-BOT, TIBAL) as Specialty Organic Synthesis Reagents (Link)
3) Brown, H. C.; Choi, Y. M.; Narasimhan, S. JOC 1982, 47, 3153
