Ethanol
Other Names:
Ethyl alcohol
General Information:
Structure:
![]()
CAS Number: 64-17-5
Molecular Weight: 46.07 g/mol
Appearance: Colorless liquid
Melting Point: -114 C
Boiling Point: 78.3 C
Density: 0.789 g/mL at 25 C
Pure ethanol (absolute, 200 proof) is very expensive due to the fact that alcohol products are heavily taxed. Denatured ethanol is considerably cheaper than absolute ethanol. The ethanol used in most labs has been denatured by adding things such as isopropanol or methanol. Denatured ethanol is not suitable for consumption.
Common Uses:
Reagent in the conversion of carboxylic acids to esters via Fisher esterification
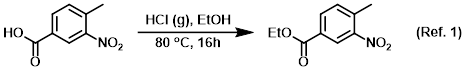
Procedure excerpt:
A solution of the SM (10 g, 55.2 mmol) in EtOH (120 mL) at -5 C was bubbled through with dry HCl gas for 10 min. The reaction mixture was stirred at 80 C for 16 h, after which time . . .
Reagent in the conversion of nitriles to esters via Pinner reactions
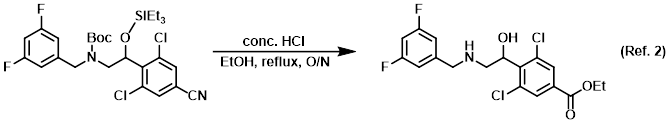
Procedure excerpt:
To a solution of the SM (0.2 g, 0.3 mmol) in EtOH (5 mL) was added conc. HCl (5 mL). The reaction mixture was stirred at reflux overnight. The mixture was quenched . . .
Solvent for reactions
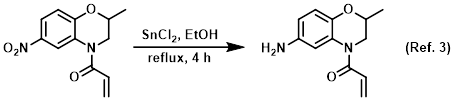
Procedure excerpt:
To a solution of the SM (700 mg, 2.81 mmol) in EtOH (5 mL) was added SnCl2 (2.8 g, 14.7 mmol) at 0 C. The resulting mixture was stirred at RT for 10 min, then reflux . . .
Solvent for silica gel column chromatography
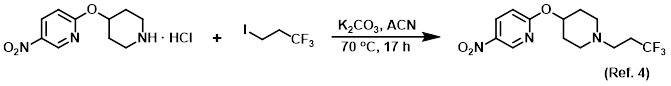
Procedure excerpt:
. . . volatiles were removed in vacuo and the crude material was purified via Prep MPLC (100 g SNAP cartridge, 0-5% EtOH/DCM) to provide . . .
Solvent for triturations
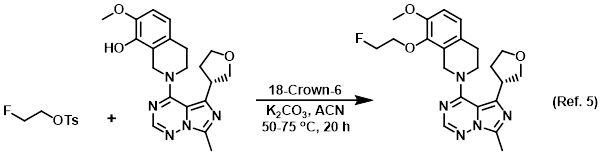
Procedure excerpt:
. . . material was combined with product from an identical reaction run at twice the scale. Trituration from cold EtOH provided the product as a white solid. . . .
Safety:
Ethanol is a flammable liquid. Drinking denatured ethanol is dangerous. For example, if it has been denatured with methanol then blindness or death could result.
References:
1) Patent Reference: WO2014149164, page 299, ![]() (23.7 MB)
(23.7 MB)
2) Patent Reference: WO2015129926, page 83, ![]() (21.5 MB)
(21.5 MB)
3) Patent Reference: WO2014149164, page 375, ![]() (23.7 MB)
(23.7 MB)
4) Patent Reference: WO2016012477, page 152, ![]() (8.1 MB)
(8.1 MB)
5) Patent Reference: WO2014177977, page 66, ![]() (6.0 MB)
(6.0 MB)
6) Wikipedia: Ethanol (link)
7) www.sigmaaldrich.com: Ethanol (link)
