Nitro Reduction
(SnCl2)
Examples:
Example 1
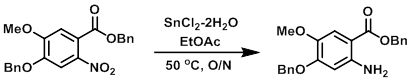
To a solution of the SM (10.9 g, 27.7 mmol) in EtOAc (100 mL) was added SnCl2-2H2O (18.7 g, 83.1 mmol). The reaction mixture was stirred at 50 C overnight. The mixture was filtered through celite, and the filtrate was washed with 10% NaHCO3. The layers were separated and the aq layer was further extracted with EtOAc. The combined organics were dried and concentrated in vacuo to provide the product as a brown solid. [9.5 g, 95%]
[Patent Reference: WO2002016361, page 35, ![]() (2.1 MB)]
(2.1 MB)]
Example 2
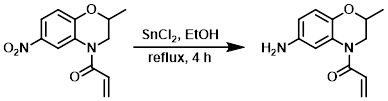
To a solution of the SM (700 mg, 2.81 mmol) in EtOH (5 mL) was added SnCl2 (2.8 g, 14.7 mmol) at 0 C. The resulting mixture was stirred at RT for 10 min, then reflux for 4 h. After completion of the reaction, ice-cold H2O was added to the reaction mixture. The residue obtained was diluted with 20% NaOH and the aq layer was extracted with EtOAc. The org layer was dried (Na2SO4) and concentrated to provide the product as a brown oily liquid. [560 mg, 91%]
[Patent Reference: WO2014149164, page 375, ![]() (23.7 MB)]
(23.7 MB)]
