Substitution (I)
(Aliphatic Amines)
Examples:
Example 1
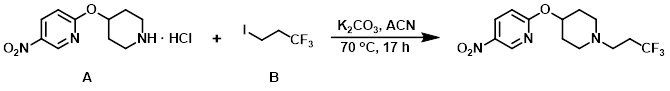
To a mixture of the amine (A) (3.3 g, 12.71 mmol) and K2CO3 (4.39 g, 31.8 mmol) suspended in ACN (54 mL) was added alkyl iodide (B) (3.13 g, 14.0 mmol). The reaction mixture was stirred at 70 C for 17 h, after which time it was cooled to RT and partitioned between EtOAc and H2O. The org layer was washed with brine and the phases were separated by the use of Whatman filter. The volatiles were removed in vacuo and the crude material was purified via Prep MPLC (100 g SNAP cartridge, 0-5% EtOH/DCM) to provide the product. [2.93 g, 69%]
[Patent Reference: WO2016012477, page 152, ![]() (8.1 MB)]
(8.1 MB)]
Example 2

A solution of the amine (A) (1.0 g, 4.1 mmol), the alkyl iodide (B) (922 mg, 4.1 mmol), and DIEA (1.06 g, 8.2 mmol) in ACN (20 mL) was stirred at reflux for 4 h. The mixture was concentrated and purified by silica gel flash chromatography (1:20 EtOAc/PE) to provide the product as a yellow oil. [620 mg, 45%]
[Patent Reference: WO2016011930, page 157, ![]() (15.7 MB)]
(15.7 MB)]