Substitution (Tosylate)
(Aromatic Alcohols)
Examples:
Example 1
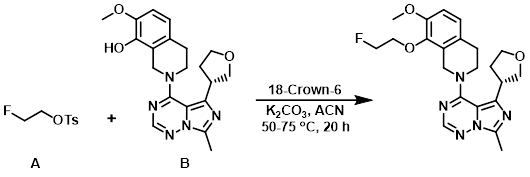
To a mixture of the alcohol (B) (200 mg, 0.524 mmol), K2CO3 (146 mg, 1.06 mmol), and ACN (7 mL) was added the tosylate (A) (126 mg, 0.577 mmol) and 18-Crown-6 (28.6 mg, 0.105 mmol). The reaction mixture was stirred at 50 C for 2 h, then 75 C for 18 h. After cooling to RT, the mixture was diluted with DCM and washed sequentially with H2O and sat aq brine. The org layer was dried (MgSO4), concentrated, and purified by silica gel chromatography (5% MeOH in EtOAc) to provide the product. The material was combined with product from an identical reaction run at twice the scale. Trituration from cold EtOH provided the product as a white solid. [553 mg, 82%]
[Patent Reference: WO2014177977, page 66, ![]() (6.0 MB)]
(6.0 MB)]
