Hydrochloric Acid
Other Names:
Muriatic acid
General Information:
Structure:
![]()
CAS Number: 7647-01-0
Molecular Weight: 36.46 g/mol
Appearance: Colorless liquid (37% w/w aq.)
Density: 1.2 g/mL (37% w/w aq)
Hydrochloric acid (HCl) is a strong mineral acid with a wide variety of uses in organic chemistry. HCl can be purchased in various different concentrations but typically it is bought in concentrated form (37-38% wt/wt, 12M). Concentrated HCl can then be used to make lower concentration stock solutions (ex. 1M, 2M, 6M).
Common Uses:
Reagent for Boc deprotections
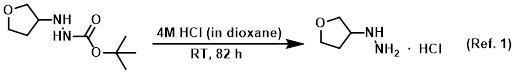
Reagent for THP deprotections
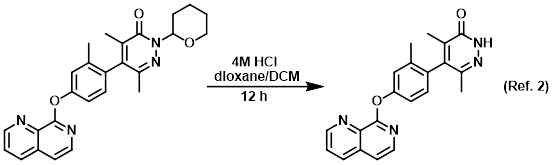
Reagent for SEM deprotections
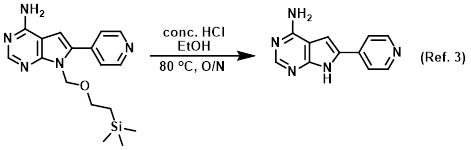
Reagent for TBS deprotections
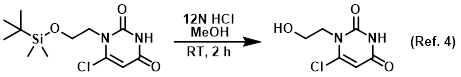
Reagent for CBz deprotections
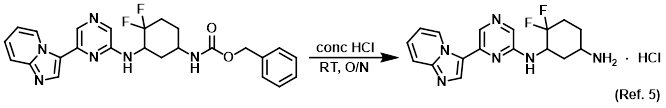
Reagent for acetyl deprotections
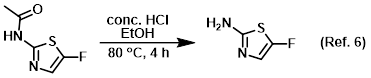
Reagent for the conversion of carboxylic acids to esters (Fisher esterification)
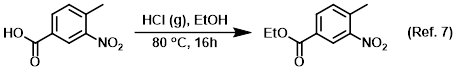
Reagent for the conversion of esters to carboxylic acids
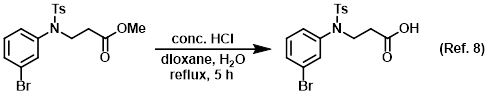
Reagent for the conversion of nitriles to carboxylic acids
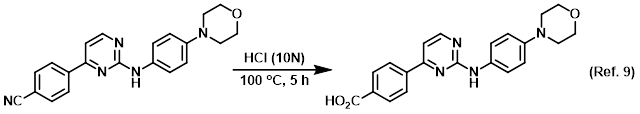
Reagent for the conversion of nitriles to esters (Pinner reaction)
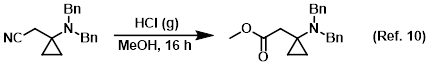
Safety:
Hydrochloric acid (HCl) is a strong acid and has the ability to cause severe damage to skin (skin contact), respiratory tract (inhalation), digestive tract (ingestion), or eyes (eye contact). Reactions of HCl with bases can be very exothermic. When making dilutions of concentrated acids always add acid to water, not water to acid.
References:
1) Patent Reference: WO2010032200, page 138, ![]() (6.2 MB)
(6.2 MB)
2) Patent Reference: WO2015162516, page 101, ![]() (5.9 MB)
(5.9 MB)
3) Patent Reference: WO2012149280, page 77, ![]() (4.1 MB)
(4.1 MB)
4) Patent Reference: WO2016011930, page 107, ![]() (15.7 MB)
(15.7 MB)
5) Patent Reference: WO2010016005, page 98, ![]() (11.3 MB)
(11.3 MB)
6) Patent Reference: WO2014201173, page 72, ![]() (19.7 MB)
(19.7 MB)
7) Patent Reference: WO2014149164, page 299, ![]() (23.7 MB)
(23.7 MB)
8) Patent Reference: WO2015089337, page 206, ![]() (17.5 MB)
(17.5 MB)
9) Patent Reference: WO2007089768, page 230, ![]() (20.6 MB)
(20.6 MB)
10) Patent Reference: WO2014149164, page 234, ![]() (23.7 MB)
(23.7 MB)
11) Wikipedia: Hydrochloric acid (link)
12) www.sigmaaldrich.com: Hydrochloric acid (link)
13) Reich, H. J.; Rigby, J. H.; Handbook of Reagents for Organic Synthesis, Acidic and Basic Reagents
