Acid to Ester
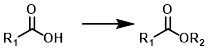
Common Conditions:
Fischer Esterification
Fisher Esterification is best suited for simple alcohols (ex. MeOH or EtOH) which can be used in large excess (as solvent). Primary and secondary alcohols work well, while tertiary alcohols and phenols work poorly. Acid sensitive substrates may not be well tolerated.[1]
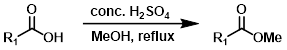
Steglich Esterification (DCC + DMAP)
Steglich Esterification is a good alternative for substrates that are acid sensitive. EDC is sometimes used in place of DCC. The reaction is commonly employed for the formation of t-butyl esters.
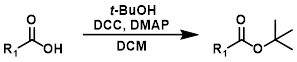
SOCl2 + MeOH
MeOH reacts with SOCl2 to provide anhydrous HCl which leads to acid catalyzed esterification (similar to Fischer Esterification). EtOH can be substituted for MeOH to make ethyl esters. Acid sensitive substrates may not be well tolerated.[2]
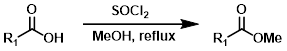
1) SOCl2 2) MeOH
Esters can be formed in a two step process where the acid chloride is first formed with SOCl2 (or oxalyl chloride) and isolated, then reacted with the alcohol (MeOH, in this case).
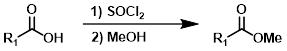
MeI
Alkylation of carboxylic acids with iodomethane (MeI) provides methyl esters. Alkylation with a large variety of electrophiles is possible, making it a useful alternative to Fisher Esterification in some cases. One drawback is the possible alkylation of other nucleophilic sites.[2]
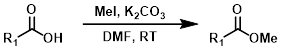
TMS-CHN2
Trimethylsilyldiazomethane (TMS-CHN2) reacts rapidly with carboxylic acids to give methyl esters. Although MeOH is recommended in these reactions, MeOH is not the methylating agent. One drawback is the possible reaction of TMS-CHN2 with other sites (ex. alcohols or phenols).[3]
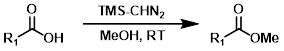
Me2SO4
Dimethyl sulfate is a powerful methylating agent. It is a useful alternative when using diazomethane or strongly acidic conditions are not possible. One drawback is the possible methylation of other nucleophilic sites.[4]
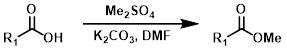
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
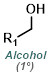 |
|||
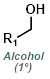 |
 |
|||
 |
 |
References:
1) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
2) Caron, S.; Practical Synthetic Organic Chemistry
3) Coates, R. M.; Denmark, S. E.; Handbook of Reagents for Organic Synthesis, Reagents, Auxiliaries, and Catalysts for C-C Bond Formation
4) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups
