Carbonylation
Examples:
Example 1
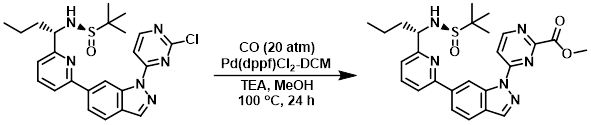
To a solution of the SM (2.0 g, 4.15 mmol) in MeOH (100 mL) was added Pd(dppf)Cl2-DCM (380 mg, 0.42 mmol) and TEA (838 mg, 8.30 mmol). The reaction mixture was stirred under an atmosphere of CO (20 atm) at 100 C for 24 h. After concentration, the residue was purified by chromatography (1:1 PE/EtOAc) to provide the product as a white solid. [1.1 g, 52%]
[Patent Reference: WO2016011390, page 320, ![]() (20.2 MB)]
(20.2 MB)]
Example 2
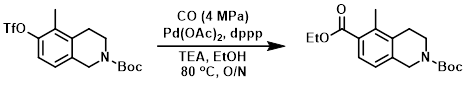
The SM (50.0 g, 127 mmol) was combined with Pd(OAc)2 (5.0 g), dppp (5.0 g), and TEA (25.7 g, 254 mmol) in EtOH (1.0 L). The reaction mixture was stirred under an atmosphere of CO (4 MPa) at 80 C overnight. The mixture was cooled to RT and the solids were removed by filtration. The filtrate was concentrated in vacuo and the residue was purified by silica gel flash chromatography (20:1 PE/EtOAc) to provide the product. [25.0 g, 62%]
[Patent Reference: WO2016014463, page 92, ![]() (6.7 MB)]
(6.7 MB)]
Example 3
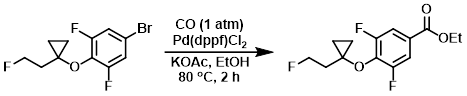
A mixture of the SM (400 mg, 1.36 mmol), KOAc (267 mg, 2.72 mmol), and Pd(dppf)Cl2 (99 mg, 0.14 mmol) in EtOH (5 mL) was stirred at 80 C under CO (1 atm) for 2 h. The mixture was filtered and the filtrate was concentrated and purified by flash chromatography column (1:30 EtOAc/PE) to provide the product as a colorless oil. [300 mg, 79.3%]
[Patent Reference: WO2016011930, page 142, ![]() (15.7 MB)]
(15.7 MB)]
Example 4
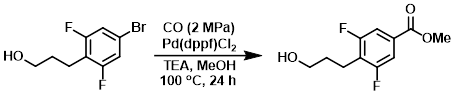
A mixture of the SM (8.10 g, 32.3 mmol), Pd(dppf)Cl2 (2.63 g, 3.2 mmol), and TEA (6.52 g, 64.5 mmol) in MeOH (100 mL) was stirred at 100 C under CO (2 MPa) for 24 h. The mixture was cooled to RT, filtered, and concentrated. The residue was dissolved in EtOAc (400 mL), washed with H2O (200 mL), brine (200 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (1:5 EtOAc/PE) to provide the product as a red oil. [2.83 g, 38%]
[Patent Reference: WO2016011930, page 159, ![]() (15.7 MB)]
(15.7 MB)]
