Ester to Alcohol
(LiBH4)
Examples:
Example 1
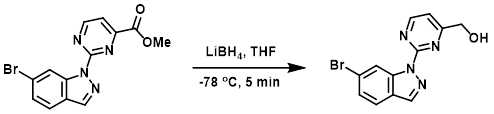
To a solution of the SM (300 mg, 0.9 mmol) in THF (15 mL) at -78 C was added dropwise LiBH4 (1.8 mL, 1.8 mmol). The reaction mixture was stirred at -78 C for 5 min, after which time it was quenched with sat aq NH4Cl. The mixture was filtered and the filtrate was extracted with EtOAc (3 x 30 mL). The combined organics were dried and concentrated to provide the product as a yellow solid. [110 mg, 48%]
[Patent Reference: WO2016011390, page 324, ![]() (20.2 MB)]
(20.2 MB)]
Example 2
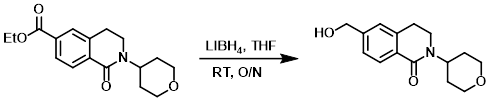
To a solution of the SM (640 mg, 2.11 mmol) in THF (20 mL) was added LiBH4 (2M in THF, 2.5 mL, 5.0 mmol). The reaction mixture was stirred at RT overnight, after which time excess reactants were consumed by the slow addition of H2O. The mixture was diluted with H2O and extracted with EtOAc. The combined organics were washed with brine, dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel flash chromatography to provide the product. [90 mg, 16%]
[Patent Reference: WO2016014463, page 119, ![]() (6.7 MB)]
(6.7 MB)]
