Toluene
Other Names:
Methylbenzene
General Information:
Structure:
![]()
CAS Number: 108-88-3
Molecular Weight: 92.14 g/mol
Appearance: Colorless liquid
Melting Point: -93 C
Boiling Point: 110-111 C
Density: 0.865 g/mL at 25 C
Toluene is a common solvent in organic chemistry. It is closely related to benzene but due to its much lower toxicity it is often used as an alternative to benzene. Xylenes are also similar to toluene and are used for many of the same reactions as toluene.
Common Uses:
Solvent for reactions
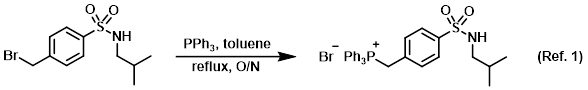
Procedure excerpt:
A mixture of the SM (1.08 g, 3.53 mmol) and PPh3 (1.39 g, 5.29 mmol) in toluene (20 mL) was stirred at reflux for 18 h. The mixture was cooled and the precipitate . . .
Solvent for azeotroping
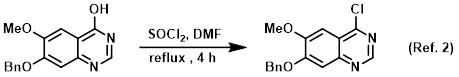
Procedure excerpt:
. . . The mixture was concentrated in vacuo. The resulting material was azeotroped with toluene to provide the product . . .
Safety:
Toluene is a flammable liquid. Toluene is much less carcinogenic than its close relative benzene. Inhalation can cause tired or euphoric effects which is why toluene is sometimes abused recreationally. Inhaling large amounts can cause neurological damage, unconsciousness, and even death.
References:
1) Patent Reference: WO2015177325, page 77, ![]() (4.3 MB)
(4.3 MB)
2) Patent Reference: WO2002016361, page 36, ![]() (2.1 MB)
(2.1 MB)
3) Wikipedia: Toluene (link)
4) www.sigmaaldrich.com: Toluene (link)
