Substitution (Br)
(Aliphatic Amines)
Examples:
Example 1
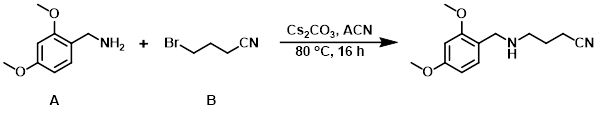
A mixture of the alkyl bromide (B) (500 mg, 3.40 mmol), the amine (A) (1.14 g, 6.80 mmol), and Cs2CO3 (2.21 g, 6.80 mmol) in ACN (10 mL) was stirred at 80 C for 16 h. After concentration, the residue was purified by silica gel column chromatography (6:1 PE/EtOAc) to provide the product as a yellow solid. [358 mg, 45%]
[Patent Reference: WO2016011390, page 121, ![]() (20.2 MB)]
(20.2 MB)]
Example 2
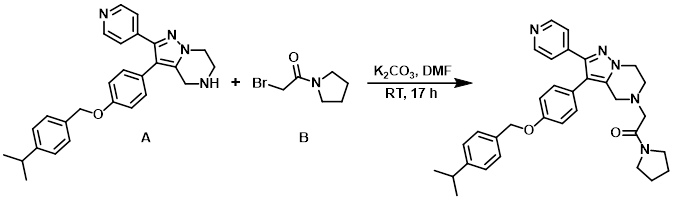
A solution of the alkyl bromide (B) (137 mg, 0.715 mmol) in DMF (0.5 mL) was added to a solution of the amine (A) (172 mg, 0.397 mmol) and K2CO3 (110 mg, 0.794 mmol) in DMF (2.5 mL) at RT. The reaction mixture was stirred at RT for 17 h, after which time H2O and EtOAc were added. The org layer was washed with brine, dried (MgSO4), and concentrated in vacuo. The resulting material (204 mg) was purified by Prep LC (12 g silica, 0-10% MeOH/DCM) to provide a sticky solid which after trituration with pentane provided the product as a pale yellow solid. [71 mg, 33 %]
[Patent Reference: WO2015144799, page 285, ![]() (18.8 MB)]
(18.8 MB)]
Example 3
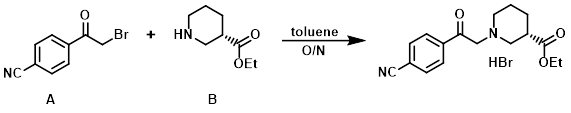
To a solution of the commercially available amine (B) (10 g, 63.6 mmol) in toluene (200 mL) was added the alkyl bromide (A) (17 g, 76 mmol). The reaction mixture was stirred overnight, after which time the precipitated solid was filtered, washed with EtOAc (3x), and dried in vacuo to provide the product as a hydrobromide salt. [15.2 g]
[Patent Reference: WO2011017578, page 105, ![]() (8.3 MB)]
(8.3 MB)]
Example 4
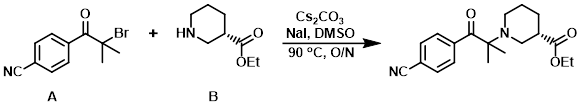
To a mixture of the amine (B) (125 mg, 0.793 mmol), Cs2CO3 (258 mg, 0.793 mmol), NaI (13 mg, 0.087 mmol), and DMSO (2 mL) was added the alkyl bromide (A) (200 mg, 0.793 mmol). The reaction mixture was stirred at 90 C overnight, after which time it was diluted with EtOAc, washed with brine, dried (MgSO4), concentrated, and purified on silica gel (EtOAc/hexane) to provide the product. [90 mg]
[Patent Reference: WO2011017578, page 154, ![]() (8.3 MB)]
(8.3 MB)]
Example 5
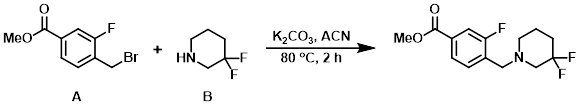
A mixture of the alkyl bromide (A) (200 mg, 0.810 mmol), the amine (B) (98 mg, 0.810 mmol), and K2CO3 (336 mg, 2.429 mmol) in ACN (20 mL) was stirred at 80 C for 2 h. The mixture was diluted with DCM and washed with H2O (2 x 30 mL). The org layer was dried (Na2SO4) and concentrated to provide the product as a light yellow oil. [270 mg, 56.7%]
[Patent Reference: WO2016011930, page 173, ![]() (15.7 MB)]
(15.7 MB)]
Example 6
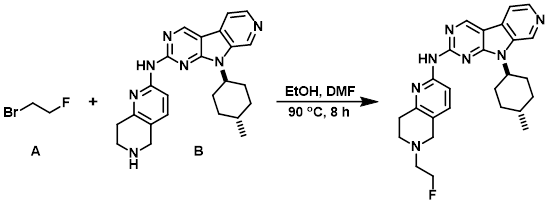
To a solution of the amine (B) (149 mg, 0.36 mmol) in absolute EtOH (4 mL) and DMF (2 mL) was added the alkyl bromide (A) (50 uL, 0.67 mmol). The resulting mixture was stirred in a pre-heated oil bath at 90 C. After 70 min, a second aliquot of the alkyl bromide (A) (50 uL, 0.67 mmol) was added. After 7.5 h of heating LCMS indicated a majority consumption of the the amine (B). The mixture was allowed to cool to RT and the volatiles were removed in vacuo. The resulting solution was diluted with DCM and washed sequentially with sat aq Na2CO3, H2O, and brine. The combined organics were dried (Na2SO4), concentrated, and purified by silica gel chromatography [10-45% (90:9:1 DCM/MeOH/NH4OH)/(DCM)]. The product fractions were combined, concentrated, and the resulting residue precipitated from DCM/hexane. The suspension was sonicated, filtered, and the resulting solids dried under high vac overnight to provide the product as an off-white solid. [35 mg, 21%]
[Patent Reference: WO2012129344, page 145, ![]() (7.3 MB)]
(7.3 MB)]
