DMSO
(Dimethyl sulfoxide)
Other Names:
Methyl sulfoxide
General Information:
Structure:
![]()
CAS Number: 67-68-5
Molecular Weight: 78.13 g/mol
Appearance: Colorless liquid
Melting Point: 16-19 C
Boiling Point: 189 C
Density: 1.10 g/mL
Dimethyl sulfoxide (DMSO) is a polar aprotic solvent that can dissolve a wide range of organic compounds. The high dissolving power of DMSO makes deuterated DMSO (DMSO-d6) a common solvent for NMR. One of the major drawbacks of DMSO is the difficulty of removal after a reaction. Its high boiling point (189 C) makes removal by rotovap very difficult. DMSO is also used as a reagent in a variety of different reactions.
Common Uses:
Reagent in the conversion of alcohols to ketones via Swern oxidation
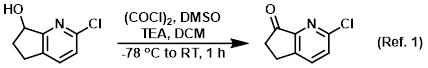
Procedure excerpt:
To a stirred solution of (COCl)2 (0.49 mL, 5.5 mmol) in DCM (12 mL) was slowly added DMSO (0.79 mL, 11.0 mmol) at -78 C. The resulting mixture was stirred . . .
Reagent in the conversion of alcohols to aldehydes via Swern oxidation
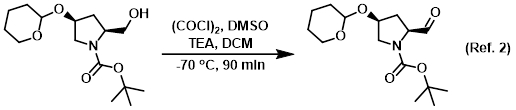
Procedure excerpt:
To a solution of (COCl)2 (19.0 g, 0.15 mol) in dry DCM (180 mL) was added dropwise a solution of dry DMSO (22.0 g, 0.30 mol) in dry DCM (60 mL) at -70 C under N2. The mixture . . .
Safety:
DMSO readily penetrates the skin.
References:
1) Patent Reference: WO2016011390, page 112, ![]() (20.2 MB)
(20.2 MB)
2) Patent Reference: WO2010016005, page 120, ![]() (11.3 MB)
(11.3 MB)
3) Wikipedia: Dimethyl sulfoxide (link)
4) www.sigmaaldrich.com: Dimethyl sulfoxide (link)
