Alcohol to Aldehyde
(Swern)
Examples:
Example 1
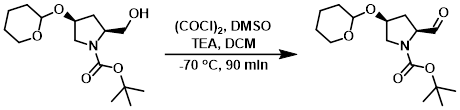
To a solution of (COCl)2 (19.0 g, 0.15 mol) in dry DCM (180 mL) was added dropwise a solution of dry DMSO (22.0 g, 0.30 mol) in dry DCM (60 mL) at -70 C under N2. The mixture was stirred at -70 C for 30 min, then was added the SM (30.0 g, 0.10 mmol) in dry DCM (120 mL). The mixture was stirred at -70 C for 90 min, then was added dry TEA (60 mL). The reaction mixture was stirred at -70 C for 90 min, after which time it was quenched with H2O (50 mL) at -70 C, then allowed to warm to RT. The layers were separated and the aq layer was further extracted with DCM (3 x 200 mL). The combined organics were washed with 5% aq citric acid (3 x 400 mL), 5% aq Na2CO3 (3 x 400 mL), and brine (3 x 400 mL). The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product as a brown liquid. [29.0 g, 97%]
[Patent Reference: WO2010016005, page 120, ![]() (11.3 MB)]
(11.3 MB)]
Example 2

The SM (300 mg, 2.14 mmol), (COCl)2 (2.0 M in DCM, 1 mL, 2 mmol), and DCM (5 mL) were combined and cooled to -78 C. DMSO (0.33 mL, 4.71 mmol) was added and the mixture was stirred for 2 h. TEA (0.66 mL, 4.71 mmol) was added and the mixture was allowed to warm to RT and stir overnight. H2O (5 mL) was added and the mixture was stirred 1 h, then extracted with DCM (2 x 50 mL). The combined organics were dried (Na2SO4) and concentrated to give the crude product as a light-brown solid that was used without further purification.
[Patent Reference: WO2015069512, page 14, ![]() (2.6 MB)]
(2.6 MB)]
