Sandmeyer
(Ar-NH2 to Ar-Br)
Examples:
Example 1
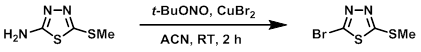
To ACN (350 mL) at RT was added CuBr2 (67 g, 300 mmol). The mixture was cooled to 0-5 C and was treated dropwise with t-BuONO (72.5 mL, 535 mmol). The mixture was stirred for 5 min, then was added the SM (35 g, 238 mmol). The reaction was stirred at RT for 2 h. EtOAc (525 mL) was added to the mixture and the reaction was quenched with 10% aq NH4Cl (350 mL) and 2% aq NH3 (175 mL). The layers were separated and the aq layer was extracted with EtOAc. The combined organics were dried and concentrated. The resulting material was purified by silica gel chromatography (eluting with 5% EtOAc/hexane) to provide the product as a yellow solid. [35 g, 70%]
[Patent Reference: WO2012129338, page 89, ![]() (12.0 MB)]
(12.0 MB)]
Example 2
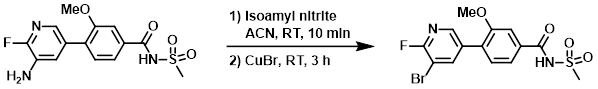
Isoamyl nitrite (17.3 g, 147.4 mmol) was added to a solution of the SM (25 g, 73.7 mmol) in ACN (160 mL), and the mixture was stirred at RT for 10 min. CuBr (21.1 g, 147 mmol) was added portionwise and the reaction mixture was stirred at RT for 3 h. The mixture was quenched with sat aq NH4Cl (100 mL). The aq layer was extracted with EtOAc (3 x 500 mL). The combined organics were washed with brine (250 mL), dried (Na2SO4), and concentrated in vacuo. The crude product was purified by silica gel column chromatography (0-10% MeOH/DCM) to provide the product. [12.0 g, 40%]
[Patent Reference: WO2015051043, page 60, ![]() (9.7 MB)]
(9.7 MB)]
Example 3
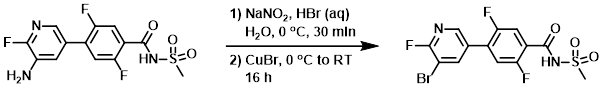
To a solution of the SM (8.00 g, 23.74 mmol) in HBr (48% in H2O, 150 mL) was added a solution of NaNO2 (16.3 g, 237 mmol) in H2O (50 mL), keeping the temp below 0 C. The reaction mixture was stirred at the same temperature for 30 min, after which time was added CuBr (34.1 g, 237 mmol) in portions. The reaction mixture was stirred at 0 C for 15 min, then allowed to stir at RT for 16 h. The mixture was diluted with EtOAc (1.0 L) and neutralized with NaHCO3. The org layer was separated, dried (Na2SO4), and concentrated to give crude material which was further purified by washing with EtOAc (100 mL) and hexane (100 mL) to provide the product. [3.0 g, 31.2%]
[Patent Reference: WO2015051043, page 107, ![]() (9.7 MB)]
(9.7 MB)]
Example 4
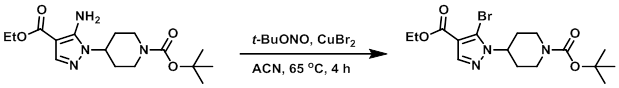
To a solution of CuBr2 (169 g, 770 mmol) in ACN (1.0 L) was slowly added t-BuONO (112 mL, 960 mmol), and the mixture was heated to 65 C. To this was added a solution of the SM (215 g, 640 mmol) in ACN (650 mL) dropwise over 30 min. After 4 h, the reaction mixture was cooled to RT and poured into 2M HCl (1.5 L) in ice. The resulting mixture was extracted with EtOAc (3x). The combined organics were washed with sat aq NaHCO3, dried (MgSO4), and concentrated. The resulting material was purified by passing it through a short plug of silica gel (eluting with 90-100% DCM/heptane) to provide the product as a yellow oil which solidified upon standing. [137 g, 53%]
[Patent Reference: WO2012069948, page 55, ![]() (3.9 MB)]
(3.9 MB)]
Example 5

To a mixture of the SM (9.50 g, 49.6 mmol), NaNO2 (5.24 g, 76 mmol), and CuBr2 (22.4 g, 100 mmol) was added ACN/H2O (1:1, 120 mL) (caution: bubbling and slight exotherm). The reaction was stirred at RT for 66 h, after which time was added aq H2SO4 (1N, 200 mL) and EtOAc (100 mL). The resulting precipitate was collected via filtration and washed with H2O and EtOAc to provide the product was a light yellow solid (7.70 g). The org layer from the filtrate was concentrated to a smaller volume and the resulting precipitate was filtered and washed with 1:1 EtOAc/heptane to provide additional product (0.4 g). [8.1 g, 75%]
[Patent Reference: WO2015162516, page 93, ![]() (5.9 MB)]
(5.9 MB)]
Example 6
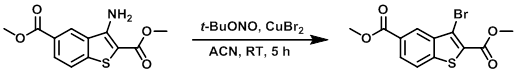
To a solution of CuBr2 (9.0 g, 40.0 mmol) in ACN at 0 C was added t-BuONO (5.2 mL, 44 mmol). The mixture was stirred for 10 min, after which time was added the SM (9.0 g, 34 mmol) in small portions at 0 C. The reaction was stirred at RT for 5 h. Upon completion, ice-cold H2O was added to the reaction mixture. The resulting solids were filtered and dried to provide the product as a yellow solid. [7.0 g, 64%]
[Patent Reference: WO2014149164, page 260, ![]() (23.7 MB)]
(23.7 MB)]
Example 7
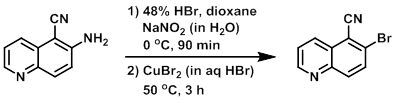
To a solution of the SM (8.0 g, 47.0 mmol) in dioxane (8.0 mL) at 0 C was added 48% HBr (95 mL), followed by the dropwise addition of a solution of NaNO2 in water (6.5 g, 94.6 mmol). The reaction was stirred at 0 C for 90 min, after which time the mixture was poured into a solution of CuBr2 (20.0 g, 142 mmol) in aq HBr at 0 C. The reaction was stirred at 50 C for 3 h. Upon completion, the reaction mixture was partitioned between EtOAc (200 mL) and H2O (100 mL). The org layer was dried (Na2SO4), concentrated, and purified by silica gel column chromatography to provide the product as a light brown solid. [6.0 g, 55%]
[Patent Reference: WO2014149164, page 269, ![]() (23.7 MB)]
(23.7 MB)]
Example 8
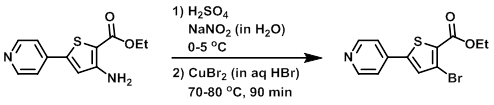
To a solution of the SM (2.0 g, 8 mmol) in H2SO4 (15 mL) at 0-5 C was added NaNO2 (667 mg, in 10 mL H2O). To this mixture was added CuBr2 (2.7 g, 12 mmol) in HBr (15 mL) at 70 C over 30 min. The resulting reaction mixture was stirred at 80 C for 1 h, after which time the mixture was diluted with H2O. The resulting solids were filtered and dried to provide the product as a brown solid. [1 g, 40%]
[Patent Reference: WO2014149164, page 286, ![]() (23.7 MB)]
(23.7 MB)]