Sulfuric Acid
General Information:
Structure:
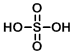
CAS Number: 7664-93-9
Molecular Weight: 98.08 g/mol
Appearance: Colorless to slightly yellow liquid
Common Uses:
Reagent in nitration reactions
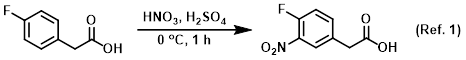
Procedure excerpt:
To a solution of the SM (3.0 g, 19.46 mmol) in H2SO4 (20 mL) at 0 C was added dropwise HNO3 (0.913 mL, 20.44 mmol). The reaction was stirred at 0 C for 1 h. The mixture . . .
Reagent in the conversion of carboxylic acids to esters via Fischer esterification
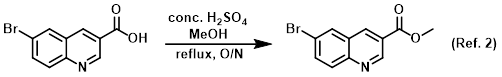
Procedure excerpt:
The SM (2.5 g, 9.92 mmol) was suspended in MeOH (40.2 mL, 991.8 mmol) and treated with conc. H2SO4 (2.64 mL, 49.59 mmol). The reaction was refluxed . . .
Reagent in the conversion of primary alcohols to carboxylic acids via Jones oxidation
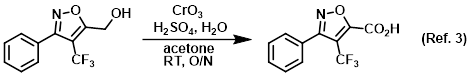
Procedure excerpt:
To an orange, homogeneous solution of CrO3 (12.4 g, 0.123 mol) in H2O (88.4 mL) at 0 C was added H2SO4 (10.8 mL) dropwise via addition funnel over 30 min . . .
Reagent in the conversion of nitriles to esters via Pinner reactions
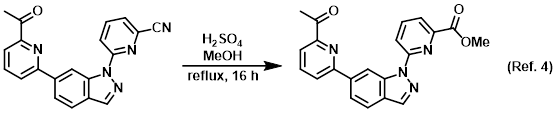
Procedure excerpt:
A solution of the SM (180 mg, 0.53 mmol) in MeOH (50 mL) and H2SO4 (2 mL) was refluxed for 16 h. After concentration, the residue was dissolved in DCM (100 mL) . . .
Reagent in Sandmeyer reactions
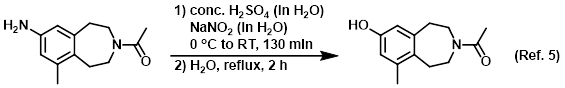
Procedure excerpt:
A 0 C solution of conc. H2SO4 (10 mL) in H2O (38 mL) was added to the SM (2.52 g, 11.5 mmol) at 0 C. Approximately 20 min later, a solution of NaNO2 (0.800 g, 11.6 mmol) . . .
Safety:
Sulfuric acid (H2SO4) is a very strong acid.
References:
1) Patent Reference: WO2013134298, page 35, ![]() (4.1 MB)
(4.1 MB)
2) Patent Reference: WO2010038081, page 116, ![]() (33.8 MB)
(33.8 MB)
3) Patent Reference: WO2011017578, page 69, ![]() (8.3 MB)
(8.3 MB)
4) Patent Reference: WO2016011390, page 201, ![]() (20.2 MB)
(20.2 MB)
5) Patent Reference: WO2016014463, page 138, ![]() (6.7 MB)
(6.7 MB)
6) Wikipedia: Sulfuric acid (link)
7) www.sigmaaldrich.com: Sulfuric acid (link)
8) Reich, H. J.; Rigby, J. H.; Handbook of Reagents for Organic Synthesis, Acidic and Basic Reagents
