Ammonia
Other Names:
Azane
General Information:
Structure:
![]()
CAS Number: 7664-41-7
Molecular Weight: 17.031 g/mol
Appearance: Colorless gas
Chemical Formula: NH3
Melting Point: -78 C
Boiling Point: -33 C
Acidity (Pka): 32.5
Basicity (Pkb): 4.75
Ammonia (NH3) has a strong pungent odor. It is a gas at room temperature and is lighter than air. Ammonia is often stored as a liquid in high pressure tanks. In organic chemistry labs ammonia is often used in other, more easy to handle forms. For example, liquid sources of ammonia are ammonium hydroxide (NH4OH) and ammonia in MeOH (typically 2N or 7N). The most common solid source of ammonia is ammonium chloride (NH4Cl).
Note: The examples below include ammonia in its commonly used forms: NH4OH, NH3 in MeOH, and NH4Cl
Common Uses:
Reagent in amide couplings
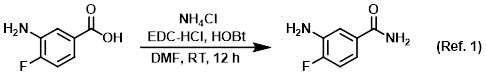
Procedure excerpt:
To a solution of the SM (1.0 g, 6.4 mmol) in DMF (5.0 mL) at 0 C was added EDC-HCl (1.2 g, 7.7 mmol), NH4Cl (1.4 g, 26.9 mmol), and HOBt (1.1 g, 8.3 mmol). The reaction . . .
Reagent in the conversion of acid chlorides to amides
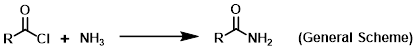
Reagent in substitution reactions
![]()
Reagent in SNAr reactions
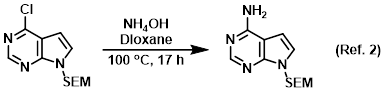
Procedure excerpt:
In a steel pressure vessel was added the SM (3.15 g, 11.1 mmol) and 40% aq NH4OH (21.6 mL, 222 mmol) in dioxane (30 mL) and the mixture was heated . . .
Reagent in reductive aminations
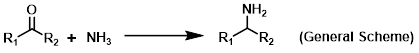
Additive in the catalytic reduction of nitriles to amines
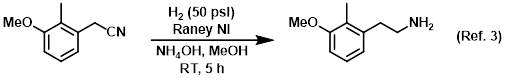
Procedure excerpt:
A mixture of the SM (180 g, 1.10 mol), Raney Ni (40.0 g), and NH4OH (250 mL) in MeOH (1.0 L) was stirred under H2 (50 psi) at RT for 5 h. The mixture . . .
Additive in silica gel chromatography
Procedure excerpt:
. . . concentrated in vacuo. The resulting crude material was purified by chromatography [330 g SiO2, 0-5% (10% NH4OH in MeOH)/(DCM)] to provide . . .
Additive in reverse phase chromatography

Procedure excerpt:
. . . heated with shaking at 90 C overnight. The crude solution was purified by reverse phase chromatography (C18, 5-95% ACN/H2O, 0.1% NH4OH) to provide . . .
Safety:
Mixing ammonia containing compounds (ex. NH3, NH4OH, or NH4Cl) and bleach (NaOCl) is very dangerous because it produces toxic by-pdts. People have died from mixing cleaning supplies that contain ammonia and bleach. Household cleaning products that contain ammonia are typically aqueous solutions (NH4OH).
References:
1) Patent Reference: WO2014149164, page 227, ![]() (23.7 MB)
(23.7 MB)
2) Patent Reference: WO2012149280, page 72, ![]() (4.1 MB)
(4.1 MB)
3) Patent Reference: WO2016014463, page 91, ![]() (6.7 MB)
(6.7 MB)
4) Patent Reference: WO2015084384, page 62, ![]() (4.2 MB)
(4.2 MB)
5) Patent Reference: WO2015051043, page 127, ![]() (9.7 MB)
(9.7 MB)
6) Wikipedia: Ammonia (link)
7) www.sigmaaldrich.com: Ammonia (link)
8) The Chlorine Institute, Sodium Hypochlorite Incompatibility Chart (link)
