SNAr (Br)
[Aliphatic Amines (2o)]
Examples:
Example 1
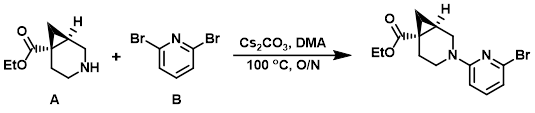
To a solution of the amine (A) (2.01 g, 11.8 mmol) in DMA (30 mL) was added the aryl bromide (B) (3.65 g, 15.4 mmol), then Cs2CO3 (8.11 g, 24.9 mmol). The reaction mixture was stirred at 100 C overnight, after which time it was cooled to RT and diluted with H2O and MTBE. The layers were separated and the aq layer was further extracted with MTBE. The combined organics were washed with brine, dried (Na2SO4), and concentrated. The resulting material was purified by silica gel flash chromatography to provide the product. [2.6 g, 68%]
[Patent Reference: WO2016014463, page 75, ![]() (6.7 MB)]
(6.7 MB)]
Example 2
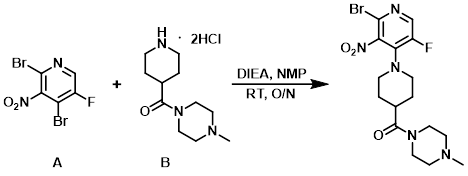
A RBF was charged with the amine (B) (16.45 g, 57.89 mmol), DIEA (31.27 mL, 179.5 mmol), and NMP (160 mL). The aryl bromide (A) (17.36 g, 57.89 mmol) was added and the reaction mixture was stirred overnight at RT under N2. Additional amine (B) (1.65 g) and DIEA (1 mL) was added, and the mixture was stirred at RT another 3 h. The mixture was diluted with EtOAc and washed with H2O (3x). The aq layer was extracted with EtOAc (3x) and the combined organics were washed with brine, dried (Na2SO4), and concentrated in vacuo. The resulting crude material was purified by chromatography [330 g SiO2, 0-5% (10% NH4OH in MeOH)/(DCM)] to provide the product as a yellow solid. [20.24 g, 81%]
[Patent Reference: WO2015084384, page 62, ![]() (4.2 MB)]
(4.2 MB)]
Example 3
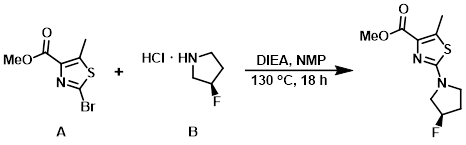
The aryl bromide (A) (188 mg, 0.796 mmol) and the amine (B) (250 mg, 1.99 mmol) were placed in a pressure vial. NMP (3.0 mL) and DIEA (0.695 mL, 3.98 mmol) were added. The vial was sealed and stirred at 130 C for 18 h. The mixture was diluted with EtOAc (100 mL), washed with H2O (3 x 50 mL), brine (50 mL), dried (Na2SO4), and concentrated to provide the crude product.
[Patent Reference: WO2016010950, page 178, ![]() (18.8 MB)]
(18.8 MB)]
