SNAr (F)
[Aliphatic Amines (2o)]
Examples:
Example 1
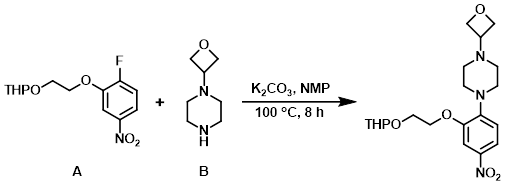
A mixture of the aryl fluoride (A) (1.550 g, 5.43 mmol), the amine (B) (772 mg, 5.43 mmol), and K2CO3 (1.126 g, 8.15 mmol) in NMP (6 mL) was stirred at 100 C for 8 h. The mixture was subjected to an aqueous workup with EtOAc extraction. The combined organics were washed with H2O (5x, to remove NMP), brine, dried (Na2SO4), and concentrated. The resulting residue was purified by column chromatography (ISCO Rf, 24 g column, 100% DCM to 60:35:5 DCM/ether/MeOH) to provide the product. [2.1 g, 94%]
[Patent Reference: WO2016010809, page 137, ![]() (11.8 MB)]
(11.8 MB)]
Example 2
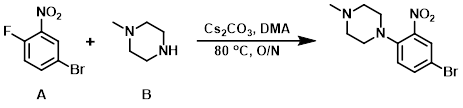
To a solution of the aryl fluoride (A) (4.0 g, 20.0 mmol) in DMA was added Cs2CO3 (11.8 g, 40 mmol), followed by the amine (B) (2.7 g, 30 mmol). The reaction mixture was stirred at 80 C overnight. After completion, the reaction mixture was diluted with H2O and extracted with EtOAc. The org layer was washed with brine, dried (Na2SO4), and concentrated in vacuo. The resulting material was purified by silica gel column chromatography to provide the product as a yellow gummy material. [3.8 g, 70%]
[Patent Reference: WO2014149164, page 333, ![]() (23.7 MB)]
(23.7 MB)]
Example 3
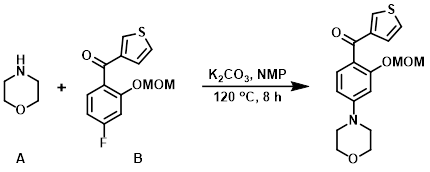
To a solution of the aryl fluoride (B) (10 g, 38 mmol) in NMP (50 mL) was added morpholine (A) (16.4 g, 188 mmol) and K2CO3 (10.4 g, 75 mmol). The reaction mixture was stirred at 120 C for 8 h. The mixture was quenched with H2O and a solution of HCl was used to adjust to pH = 5. The mixture was extracted with DCM and concentrated in vacuo. The resulting material was purified by silica gel column chromatography to provide the product. [7.2 g, 60%]
[Patent Reference: WO2015140133, page 141, ![]() (11.7 MB)]
(11.7 MB)]
Example 4
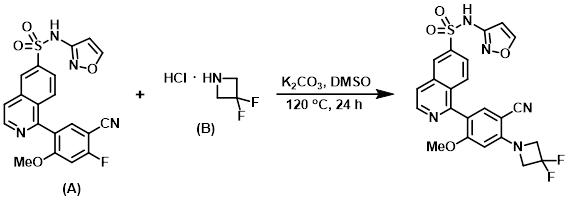
A vial was charged with the aryl fluoride (A) (0.060 g, 0.128 mmol), the amine (B) (0.056 mL, 0.429 mmol), and K2CO3 (0.053 g, 0.384 mmol). DMSO (0.641 mL) was added and the reaction mixture was stirred at 120 C for 24 h. The mixture was diluted with EtOAc and washed with H2O. The aq layer was extracted with EtOAc, and the combined organics were dried (Na2SO4), concentrated, and purified by Gilson HPLC (25-70% ACN:H2O w/ 0.1% TFA modifier). The product fractions were partitioned between EtOAc and H2O. The aq layer was extracted with EtOAc, and the combined organics were dried (Na2SO4) and concentrated to provide the product as a bright yellow solid. [0.089 g, 83%]
[Patent Reference: WO2014201173, page 144, ![]() (19.7 MB)]
(19.7 MB)]
Example 5

To a vial charged with the aryl fluoride (B) (50 mg, 0.127 mmol) was added TEA (38.5 mg, 0.38 mmol), DMSO (0.507 mL), and the amine (A) (0.317 mmol). The reaction mixture was heated with shaking at 90 C overnight. The crude solution was purified by reverse phase chromatography (C18, 5-95% ACN/H2O, 0.1% NH4OH) to provide the product. [30 mg, 44%]
[Patent Reference: WO2015051043, page 127, ![]() (9.7 MB)]
(9.7 MB)]
