SNAr (Cl)
[Aliphatic Amines (1o)]
Examples:
Example 1
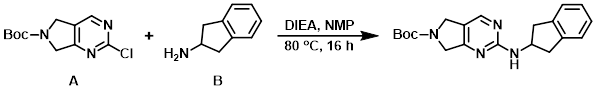
To a 15 C solution of the aryl chloride (A) (220 g, 860.37 mmol) and the amine (B) (137.7 g, 1.03 mol) in NMP (3.6 L) was added DIEA (450 mL, 2.58 mol). The resulting mixture was stirred at 80 C for 16 h, after which time it was cooled to 30 C and transferred into H2O (5 L) at RT. The resulting solids were filtered and the filter cake was rinsed with H2O (2 x 300 mL). The solid was slurried in EtOAc (350 mL) for 45 min at 15 C. The mixture was filtered, rinsing with 15 C EtOAc (2 x 250 mL), and dried to provide the product as an off-white solid. [226 g, 75%]
[Patent Reference: WO2014110000, page 19, ![]() (2.6 MB)]
(2.6 MB)]
Example 2
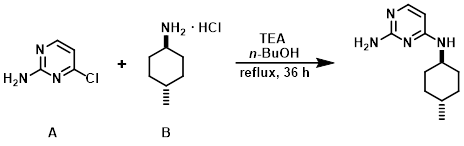
A mixture of the aryl chloride (A) (1 Kg, 7.72 mol), the amine (B) (1.5 Kg, 10.03 mol), TEA (3.23 L, 23.2 mol), and n-BuOH (8 L) were stirred at reflux for 36 h. Upon completion, the reaction mixture was cooled to RT, diluted with H2O (8 L), and extracted with EtOAc (2 x 10 L). The combined organics were dried (Na2SO4) and concentrated to provide the product. [1.77 Kg]
[Patent Reference: WO2012129344, page 123, ![]() (7.3 MB)]
(7.3 MB)]
Example 3
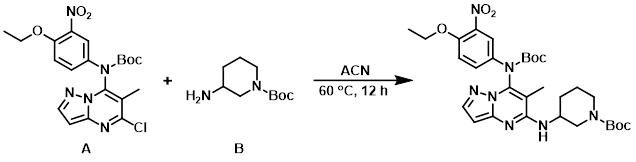
To a solution of the aryl chloride (A) (200 mg, 0.4 mmol) in ACN (3 mL) was added the amine (B) (268 mg, 1.3 mmol). The reaction mixture was stirred at 90 C for 12 h, after which time the mixture was partitioned between EtOAc (100 mL) and H2O (50 mL). The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product as a white solid. [180 mg, 70%]
[Patent Reference: WO2014149164, page 290, ![]() (23.7 MB)]
(23.7 MB)]
Example 4
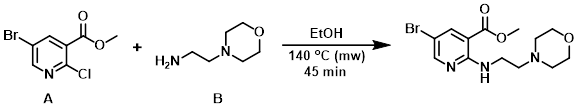
A suspension of the aryl chloride (A) (0.5 g, 2.00 mmol) and the amine (B) (0.390 mL, 2.99 mmol) in EtOH (3 mL) was heated in a microwave reactor at 140 C for 45 min. The mixture was concentrated in vacuo. The resulting residue was diluted with H2O, treated with saturated aq NaHCO3, and extracted with EtOAc. The org layer was washed with brine, dried (Na2SO4), concentrated onto silica, and purified by silica gel column chromatography (0-16% MeOH/DCM) to provide the product. [582 mg]
[Patent Reference: WO2010038081, page 259, ![]() (33.8 MB)]
(33.8 MB)]
Example 5
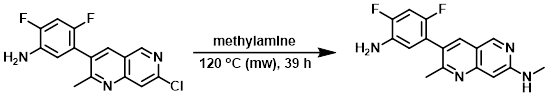
A solution of the SM (0.55 g, 1.799 mmol) in methylamine (33% in EtOH, 10 mL, 1.799 mmol) was heated via microwave at 120 C for 12 h. Additional methylamine (33% in EtOH, 2 mL) was added and the mixture was heated via microwave at 120 C for 12 h. Additional methylamine (33% in EtOH, 2 mL) was again added and the mixture was heated via microwave at 120 C for 15 h. The mixture was diluted with EtOAc, washed with sat aq NaHCO3 (2x), dried, and concentrated to provide the product. [0.53 g, 98%]
[Patent Reference: WO2013134298, page 50, ![]() (4.1 MB)]
(4.1 MB)]
Example 6
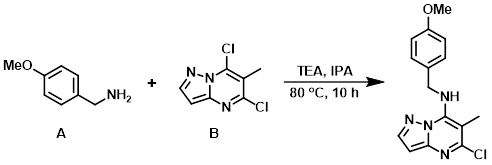
To a solution of the aryl chloride (B) (1.0 g, 5.0 mmol) in IPA (50 mL) at RT was added the amine (A) (817 mg, 6.0 mmol) and TEA (989 mg, 10.0 mmol). The reaction mixture was stirred at 80 C for 10 h. After completion, the reaction mixture was concentrated in vacuo and partitioned between EtOAc (100 mL) and H2O (50 mL). The org layer was dried (Na2SO4) and concentrated in vacuo. The resulting material was triturated in n-pentane to provide the product as an off-white solid. [1.3 g, 87%]
[Patent Reference: WO2014149164, page 296, ![]() (23.7 MB)]
(23.7 MB)]
Example 7
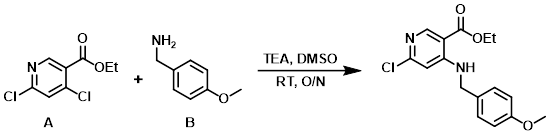
A mixture of the aryl chloride (A) (16 g, 73.1 mmol), the amine (B) (10 g, 73.1 mmol), and TEA (15.2 g, 146 mmol) in DMSO (150 mL) was stirred at RT overnight. The mixture was diluted with EtOAc and washed with H2O (2x), then brine (1x), dried (MgSO4), and concentrated to provide the product. [21 g, 90%]
[Patent Reference: WO2013134298, page 39, ![]() (4.1 MB)]
(4.1 MB)]
Example 8
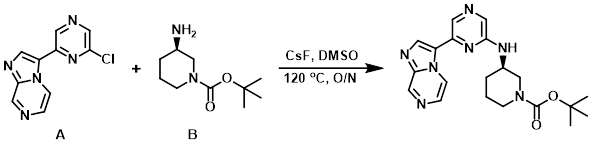
A mixture of CsF (178 mg, 1.17 mmol), the aryl chloride (A) (100 mg, 0.43 mmol), and the amine (B) (130 mg, 0.65 mmol), in DMSO (0.9 mL) was heated to 120 C overnight. The crude product was filtered and purified by HPLC (H2O/ACN with AcOH).
[Patent Reference: WO2010016005, page 85, ![]() (11.3 MB)]
(11.3 MB)]
Example 9
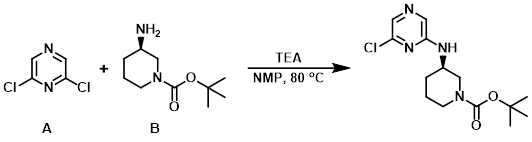
A mixture of the amine (B) (200 mg, 1 mmol), the aryl chloride (A) (149 mg, 1 mmol), and TEA (129 mg 1 mmol), in NMP (2 mL) was heated to 80 C. The reaction mixture was concentrated in vacuo and the crude material was purified by silica gel flash chromatography (30 -100% EtOAc/hexane) to provide the product as a clear oil. [187 mg, 60 %]
[Patent Reference: WO2010016005, page 80, ![]() (11.3 MB)]
(11.3 MB)]
Example 10
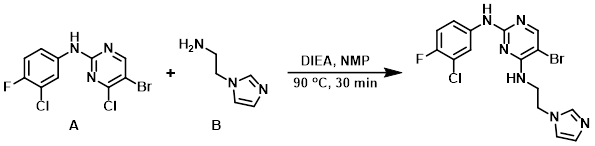
To the aryl chloride (A) (500 mg, 1.48 mmol) in NMP (5 mL) was added DIEA (3.04 mL, 1.78 mmol) and the amine (B) (181 mg, 1.63 mmol) under inert atmosphere. The reaction was stirred at 90 C for 30 min. After completion, the reaction mixture was quenched with H2O and extracted with EtOAc. The org layer was washed with brine and dried (Na2SO4). The crude was purified by column chromatography (2% MeOH/CHCl3) to provide the product. [160 mg, 26%]
[Patent Reference: WO2010038081, page 89, ![]() (33.8 MB)]
(33.8 MB)]
