Nitro Reduction
(Fe)
Examples:
Example 1

To a solution of the SM (70 g, 386 mmol) in AcOH (100 mL) and absolute EtOH (400 mL) was slowly added iron powder (40 g). The reaction was cooled in an ice-H2O bath and treated with conc. HCl (1 mL). The addition was exothermic. The reaction was heated to reflux for 20 min, after which time it was allowed to cool to room temperature. The mixture was filtered and the filtrate concentrated to a thick oil. The resulting oil was partitioned between EtOAc (500 mL) and H2O (200 mL) and basified to pH 10 using 6N NaOH. The mixture was filtered through celite. The org layer was separated and washed with sat aq NaHCO3 (2 x 100 mL), H2O (2 x 100 mL), brine (100 mL), dried (Na2SO4), and concentrated to provide the product. [37.2 g, 64%]
[Patent Reference: WO2007117607, page 306, ![]() (12.9 MB)]
(12.9 MB)]
Example 2
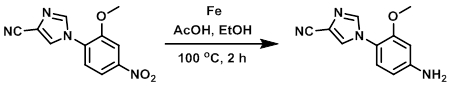
To a solution of the SM (689 mg, 2.82 mmol) in EtOH (15 mL) was added AcOH (7.5 mL) and Fe (630 mg, 11.29 mmol). The resulting mixture was brought to 100 C and stirred 2 h. The reaction was then diluted with H2O and brought to pH 8 by the addition of 1 N aq NaOH. The mixture was extracted with EtOAc (3 x 5 mL). The combined organics were washed with H2O (5 mL), brine (5 mL), dried (MgSO4), and concentrated to provide the product.
[Patent Reference: WO2011014535, page 39, ![]() (17.3 MB)]
(17.3 MB)]
Example 3
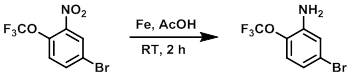
To a solution of the SM (2.0 g, 7.0 mmol) in AcOH (10 mL) at 0-10 C was added Fe powder (1.0 g, 17.9 mmol). The reaction mixture was allowed to stir at RT for 2 h. After completion, the reaction mixture was concentrated in vacuo and the resulting material was diluted with H2O and basified with sat aq NaHCO3. The mixture was extracted with EtOAc (2 x 50 mL), dried (Na2SO4), and concentrated to provide the product as a brown gummy solid. [1.2 g, 70%]
[Patent Reference: WO2014149164, page 280, ![]() (23.7 MB)]
(23.7 MB)]
Example 4
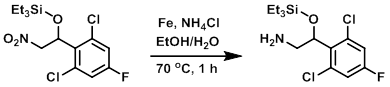
To a solution of the SM (15.0 g, 40.7 mmol) in 4:1 EtOH/H2O (60 mL) was added Fe powder (22.7 g, 407.6 mmol) and NH4Cl (21.8 g, 407.6 mmol). The reaction mixture was stirred at 70 C for 1 h. The mixture was filtered through a pad of celite and the pad was washed with EtOAc (3 x 150 mL). The filtrate was concentrated and the resulting material was suspended in H2O (100 mL) and extracted with EtOAc (3 x 100 mL). The combined organics were washed with brine (100 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel column chromatography (5% MeOH/DCM) to provide the product as a colorless oil. [13.0 g, 94%]
[Patent Reference: WO2015129926, page 74, ![]() (21.5 MB)]
(21.5 MB)]
