SNAr (F)
(Aliphatic Alcohols)
Examples:
Example 1
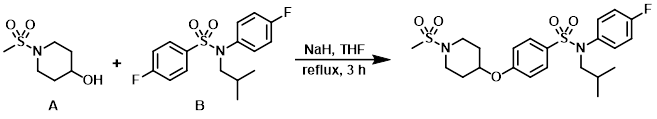
To a solution of the alcohol (A) (61 mg) in anhydrous THF (5 mL) at RT was added NaH (60% dispersion on mineral oil, 15 mg, 369 umol). The mixture was stirred at RT for 30 min. Then was added the aryl fluoride (B) (100 mg, 308 umol) and the reaction mixture was heated at reflux for 3 h. The mixture was allowed to cool to RT and H2O was added. The mixture was extracted with EtOAc, concentrated, purified by Prep reverse-phase HPLC, and freeze dried to provide the product. [51.8 mg]
[Patent Reference: WO2015177325, page 57, ![]() (4.3 MB)]
(4.3 MB)]
Example 2
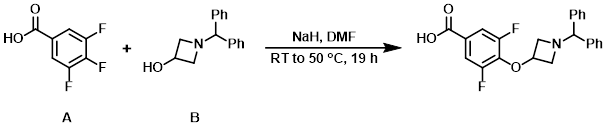
To a solution of the aryl fluoride (A) (50 g, 284 mmol) and the alcohol (B) (68 g, 284 mmol) in DMF (1 L) was added NaH (60% dispersion in mineral oil, 34 g, 852 mmol) at 0 C. The reaction mixture was stirred at RT for 16 h, then 50 C for 3 h. The mixture was poured into ice-H2O, acidified to pH = 3 with conc. HCl, and filtered. The resulting solids were triturated with PE (300 mL) and filtered to provide the product as a white solid. [88 g, 79%]
[Patent Reference: WO2016011930, page 155, ![]() (15.7 MB)]
(15.7 MB)]
Example 3
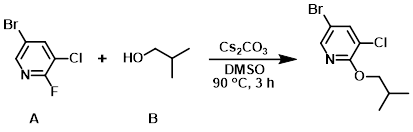
To a solution of the aryl fluoride (A) (5.0 g, 23.7 mmol) and the alcohol (B) (5.28 g, 71.3 mmol) in DMSO (100 mL) was added Cs2CO3 (23.0 g, 71.3 mmol). The reaction mixture was stirred at 90 C for 3 h, after which time it was allowed to cool to RT. The mixture was diluted with H2O (500 mL) and the aq mixture was extracted with ether (2 x 500 mL). The combined organics were dried (Na2SO4) and concentrated. The crude material was purified by silica gel column chromatography (0-5% EtOAc/hexane) to provide the product as a colorless oil. [5.0 g, 86%]
[Patent Reference: WO2015051043, page 45, ![]() (9.7 MB)]
(9.7 MB)]
Example 4
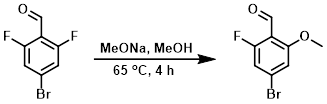
To a solution of the SM (106 g, 482 mmol) in MeOH (150 mL) at RT was added MeONa (26.0 g, 482 mmol). The reaction mixture was stirred at 65 C for 4 h. The mixture was concentrated in vacuo and the resulting residue was purified by silica gel column (10:1 PE/EtOAc) to provide the product as a light yellow solid. [69 g, 62%]
[Patent Reference: WO2016011390, page 269, ![]() (20.2 MB)]
(20.2 MB)]
Example 5
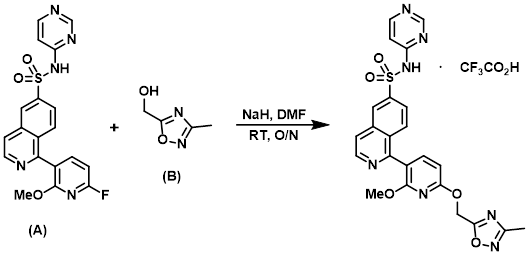
The aryl fluoride (A) (40 mg, 0.097 mmol), the alcohol (B) (110 mg, 0.97 mmol), NaH (60% wt, 39 mg, 0.97 mmol), and DMF (0.28 mL) were combined in a reaction vial and stirred at RT overnight. The reaction mixture was purified by reverse phase HPLC (Waters Xbridge C18 19 x 100 mm 10 micron column, gradient using 0.1% TFA in H2O/ACN) to provide the product as the TFA salt. [13.9 mg, 23%]
[Patent Reference: WO2014201173, page 177, ![]() (19.7 MB)]
(19.7 MB)]
