Nitrile to Amine
(BH3-THF)
Examples:
Example 1
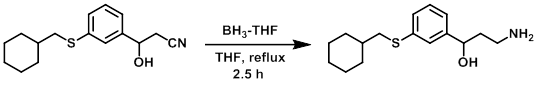
To a stirred solution of the SM (0.660 g, 2.40 mmol) in dry THF under argon was added BH3-THF (1M in THF, 5 mL, 5 mmol). The reaction was stirred at reflux for 2.5 h, after which time it was allowed to cool to RT. To the mixture was carefully added saturated NaHCO3 followed by brine. The mixture was vigorously stirred. The org layer was separated, concentrated, and purified by flash chromatography [4% (7N NH3 in MeOH)/(DCM)] to provide the product as a colorless oil. [0.480 g, 72%] [UK Pat App GB2463151A, page 123]
Example 2
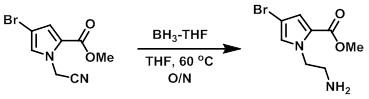
To a solution of the SM (242 mg, 1 mmol) in THF (10 mL) at RT was added dropwise BH3-THF complex (1.0 M in THF, 5 mL). The reaction was stirred at 60 C overnight. After completion, the reaction mixture was cooled to 0 C and quenched with sat aq NaHCO3. The mixture was extracted with EtOAc and the organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. The resulting material was taken forward without further purification. LCMS indicated clean product.
[Patent Reference: WO2014149164, page 315, ![]() (23.7 MB)]
(23.7 MB)]
Example 3
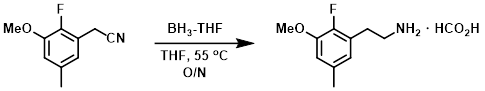
To a solution of the SM (0.92 g, 5.2 mmol) in THF was added, dropwise via syringe, a solution of BH3-THF (1.0M, 11 mL, 11 mmol). The reaction mixture was stirred at 55 C overnight. The resulting mixture was cooled to RT and excess reactants were consumed by the addition of H2O (3 mL). After 5 min, conc. HCl (3 mL) was added. After stirring for 1 h, H2O (10 mL) and solid NaOH were added until the mixture became alkaline. DCM (50 mL) was then added and the layers were separated with a hydrophobic frit. The org phase was dried (Na2SO4) and concentrated. The crude residue was purified by reverse phase flash chromatography (using a ACN/H2O mixture with 0.1% formic acid). The resulting eluent was concentrated in vacuo and azeotroped with MTBE to provide the product as a formate salt. [777 mg, 66%]
[Patent Reference: WO2016014463, page 111, ![]() (6.7 MB)]
(6.7 MB)]
