Ester to Acid
(NaOH + H2O/MeOH)
Examples:
Example 1
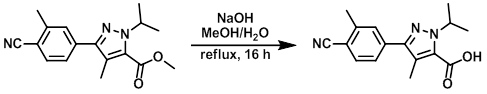
To a solution of the SM (2.31 g, 7.77 mmol) in MeOH (50 mL) and H2O (2.8 mL) was added NaOH (0.46 g, 11.7 mmol). The mixture was heated to reflux for 16 h, then cooled and concentrated in vacuo. The crude was diluted with H2O (100 mL) and acidified with an aq solution of 2M HCl. This aq mixture was extracted with EtOAc (2 x 200 mL) and the combined org extracts were washed with brine (400 mL), dried (MgSO4), and concentrated in vacuo to provide to product as a yellow solid. [2.2 g, 100%]
[Patent Reference: WO2010032200, page 135, ![]() (6.2 MB)]
(6.2 MB)]
Example 2
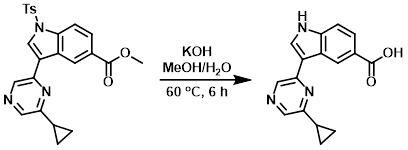
An orange-brown suspension of the SM (353 mg, 0.790 mmol) and KOH (310 mg, 5.53 mmol) in MeOH (6 mL) and H2O (1.2 mL) was heated at 60 C for 6 h. The mixture was then cooled to RT and MeOH was removed in vacuo. 1N aq HCl (4 mL) was added to the resulting slurry, and the precipitated solids were filtered, washed with H2O (10 mL), and dried in vacuo to provide the product as a yellow solid. [209 mg, 95%]
[Patent Reference: WO2012129338, page 135, ![]() (12.0 MB)]
(12.0 MB)]
Example 3
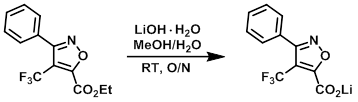
A mixture of the SM (0.085 g, 0.298 mmol) and LiOH hydrate (0.013 g, 0.298 mmol) in MeOH (2.0 mL) and H2O (1.0 mL) was stirred at RT overnight. The reaction mixture was concentrated to dryness to provide the product as a pale yellow solid. [0.079 g, 100%]
[Patent Reference: WO2011017578, page 74, ![]() (8.4 MB)]
(8.4 MB)]
Example 4

To a solution of the SM (1.2 g, 4.21 mmol) in EtOH (20 mL) was added a predissolved solution of NaOH (1.00 g, 25.1 mmol) in H2O (5.00 mL). The reaction mixture was stirred at RT overnight. The reaction mixture was then concentrated in vacuo and acidified with 1N HCl. The aq mixture was extracted with EtOAc but not all product extracted. The aq layer was brought to pH 5 using NH4OH and NH4Cl. The mixture was then again extracted with EtOAc. The combined organics were dried (MgSO4) and concentrated to provide the product. [140 mg, 13%]
[Patent Reference: WO2011017578, page 183, ![]() (8.4 MB)]
(8.4 MB)]
Example 5
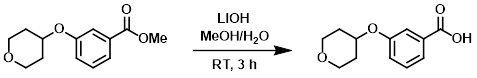
A solution of the SM (18.1 g, 76.6 mmol), LiOH (9.2 g, 383 mmol), MeOH (80 mL), and H2O (5 mL) was stirred at RT for 3 h. The reaction mixture was filtered and the filtrate was adjusted to pH 2-3 with 1M HCl. The resulting solution was extracted with EtOAc (2 x 80 mL) and concentrated to provide the product as a yellow solid which was taken forward without further purification. [14.3 g, 84%]
[Patent Reference: WO2015140133, page 118, ![]() (11.7 MB)]
(11.7 MB)]
