Substitution (I)
(Aromatic Alcohols)
Examples:
Example 1
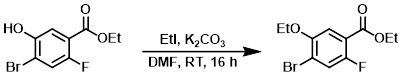
To a solution of the SM (40 g, 152.1 mmol) in DMF (400 mL) was added K2CO3 (63 g, 456 mmol) and EtI (24.3 mL, 304.2 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was concentrated in vacuo, diluted with H2O (200 mL), and extracted with EtOAc (2 x 200 mL). The combined organics were washed with H2O (100 mL), dried (Na2SO4), and concentrated to provide the product as an off-white solid. [43 g, 95%]
[Patent Reference: WO2015051043, page 98, ![]() (9.7 MB)]
(9.7 MB)]
Example 2
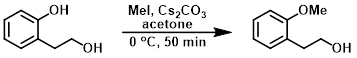
To a stirred suspension of the SM (5 g, 36.2 mmol) and Cs2CO3 (38.9 g, 108.7 mmol) in acetone (100 mL) was added MeI (6.2 g, 43.4 mmol) at 0 C. The reaction mixture was stirred at 0 C for 50 min. The mixture was filtered and the filtrate was concentrated in vacuo. The resulting crude material was partitioned between EtOAc and H2O. The org layer was dried (Na2SO4) and concentrated to provide the product as a yellow solid. [4.5 g, 82%]
[Patent Reference: WO2015140133, page 108, ![]() (11.7 MB)]
(11.7 MB)]
Example 3
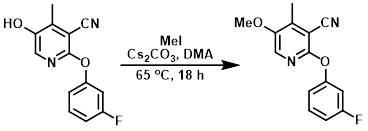
To a mixture of the SM (0.7 g, 2.86 mmol) in DMA (10 mL) was added Cs2CO3 (2.79 g, 8.59 mmol) and MeI (0.89 mL, 14.34 mmol) at RT under N2. The reaction mixture was stirred at 65 C for 18 h. The mixture was cooled to RT, poured into ice-cold H2O, and extracted with EtOAc (3 x 15 mL). The combined organics were washed with brine, dried (Na2SO4), and concentrated in vacuo to give crude product. The crude material was purified by silica gel column chromatography (7% EtOAc/hexane) to provide the product. [0.7 g, 94%]
[Patent Reference: WO2016021742, page 114, ![]() (7.7 MB)]
(7.7 MB)]
Example 4
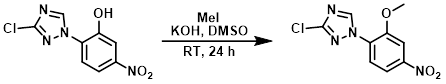
MeI (0.860 mL, 13.82 mmol) was added to a mixture of the SM (1.33 g, 5.53 mmol), KOH (388 mg, 6.91 mmol), and DMSO (25 mL). The mixture was left to stir at RT for 24 h. The reaction mixture was poured into H2O (250 mL) and extracted with EtOAc (3 x 100 mL). The combined extracts were washed with H2O, brine, dried (Na2SO4), and concentrated in vacuo. The crude residue was purified by silica gel column chromatography (0-1% MeOH/CHCl3) to provide the product as a light yellow solid. [924 mg, 66%]
[Patent Reference: WO2011014535, page 46, ![]() (17.3 MB)]
(17.3 MB)]
