Substitution (I)
(Amides)
Examples:
Example 1
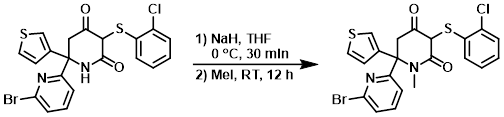
To a solution of the SM (1 g, 2 mmol) in anhydrous THF (20 mL) at 0 C was added NaH (288 mg, 12 mmol). The mixture was stirred at 0 C for 30 min. Iodomethane (1.65 g, 12 mmol) was added and the reaction mixture was stirred at RT for 12 h. The mixture was quenched with H2O, dried, and concentrated. The crude residue was purified by silica gel chromatography (10-50% EtOAc/hexane) to provide the product as a yellow solid. [475 mg, 46%]
[Patent Reference: WO2015140133, page 94, ![]() (11.2 MB)]
(11.2 MB)]
Example 2
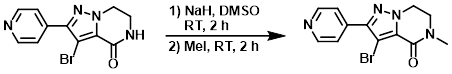
NaH (60%, 655 mg, 216 mmol) was added to a suspension of the SM (4.00 g, 13.6 mmol) in DMSO (50 mL) at RT under N2. The mixture was stirred for 2 h. MeI (1.02 mL, 16.4 mmol) was added and the reaction mixture was stirred an additional 2 h. The mixture was poured into H2O and extracted with EtOAc. The org layer was dried (MgSO4) and concentrated to provide the product as a yellow solid. [5.00 g, quant.]
[Patent Reference: WO2015144799, page 132, ![]() (18.8 MB)]
(18.8 MB)]
Example 3
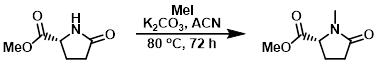
To a mixture of the SM (3.5 g, 24.5 mmol), K2CO3 (6.8 g, 49 mmol), in ACN (100 mL) was added dropwise MeI (6.1 mL, 114 mmol) at 0 C. After stirring at 80 C for 72 h, the reaction mixture was concentrated and the residue was partitioned between H2O (100 mL) and DCM (350 mL). The org layer was dried (Na2SO4) and concentrated to provide the product as a yellow oil. [3.2 g, 84%]
[Patent Reference: WO2016100281, page 231, ![]() (10.3 MB)]
(10.3 MB)]
Example 4
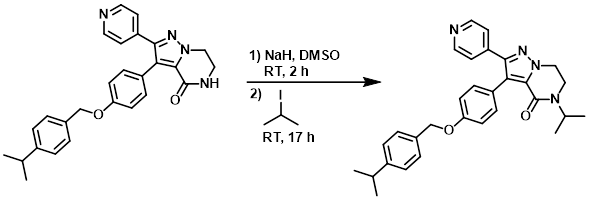
NaH (60%, 41 mg, 1 mmol) was added slowly to a suspension of the SM (0.3 g, 0.68 mmol) in DMSO (4 mL) at RT under N2. The mixture was stirred for 2 h, then was added 2-iodopropane (0.137 mL, 1.4 mmol). The reaction mixture was stirred for 17 h. H2O was added and the mixture was filtered and washed with H2O. The resulting material was dissolved in DCM, dried (MgSO4), and concentrated in vacuo to give 0.34 g of material which was purified by prep LC (100% DCM to 95% DCM/5% MeOH/0.1% NH4OH). The product fractions were combined and concentrated to give 203 mg of product. This batch was combined with another batch (125 mg) and crystallized from ether, filtered, and dried to provide the product. [289 mg, 44% (global yield)]
[Patent Reference: WO2015144799, page 145, ![]() (18.8 MB)]
(18.8 MB)]
Example 5
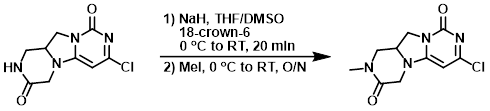
NaH (49.9 mg, 1.247 mmol) was added to a suspension of the SM (200 mg, 0.831 mmol) and 18-crown-6 (10.39 mg, 0.042 mmol) in THF (14 mL) and DMSO (2 mL) at 0 C. The mixture was stirred at RT for 20 min. The mixture was cooled to 0 C and treated with MeI (0.083 mL, 1.330 mmol), then stirred at RT overnight. The mixture was quenched with H2O and concentrated. The residue was purified using CombiFlash Rf 200 to provide the product as a yellow solid. [200 mg]
[Patent Reference: WO2016011930, page 117, ![]() (15.7 MB)]
(15.7 MB)]