SNAr (Cl)
(Aromatic Amines)
Examples:
Example 1
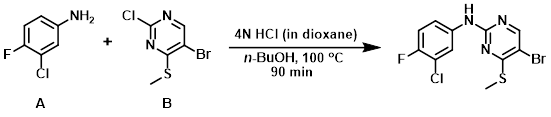
The aryl chloride (B) (30 g, 125 mmol) and the amine (A) (18.22 g, 125 mmol) were suspended in n-BuOH (300 mL). The mixture was then treated with 4 N HCl in dioxane (25 mL, 100 mmol) and refluxed at 100 C for 90 min under N2. The reaction was cooled to RT, diluted with diethyl ether, and the resulting solid was filtered and dried to provide the product as a pale yellow solid. [29 g, 67%]
[Patent Reference: WO2010038081, page 89, ![]() (33.8 MB)]
(33.8 MB)]
Example 2
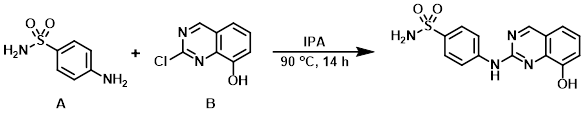
To a 0.3M solution of the aryl chloride (B) (1.0 eq) in IPA was added the amine (A) (1.0 eq). The reaction was stirred at 90 C for 14 h, after which time the HCl salt was collected by vacuum filtration. The solids were stirred in aqueous NaHCO3, filtered, washed with H2O, and dried in a desiccator to provide the desired product. [93%]
[Patent Reference: WO2007117607, page 305, ![]() (12.9 MB)]
(12.9 MB)]
Example 3
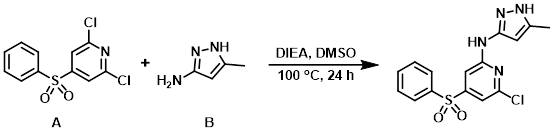
To a solution of the aryl chloride (A) (5.0 g, 17.35 mmol) and the amine (B) (1.85 g, 19.09 mmol) in DMSO (15 mL) was added DIEA (5.7 mL, 34.7 mmol). The reaction mixture was stirred at 100 C for 18 h. Additional amine (1.00 g, 10.37 mmol) was added and stirring was continued for another 6 h. The mixture was partitioned between EtOAc and sat aq NH4Cl. The layers were separated and the aq layer was further extracted with EtOAc. The combined organics were washed with H2O, brine, dried (MgSO4), and concentrated. The crude material was purified by flash chromatography (0-100% EtOAc/heptane) to provide the product as a pale yellow solid. [3.76 g, 62%]
[Patent Reference: WO2016001341, page 87, ![]() (9.1 MB)]
(9.1 MB)]
Example 4
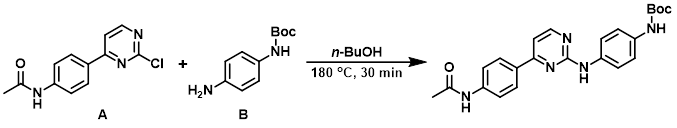
A mixture of the aryl chloride (A) (500 mg, 2.0 mmol), the amine (B) (687 mg, 3.3 mmol), and n-BuOH (5 mL) was stirred at 180 C for 30 min. The crude reaction mixture was taken directly to the deprotection step.
[Patent Reference: WO2007089768, page 223, ![]() (20.6 MB)]
(20.6 MB)]
Example 5
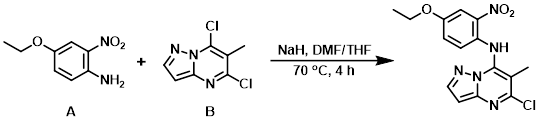
To a solution of NaH (750 mg, 0.5 mmol) in DMF (5.0 mL) at 0 C was added the aryl chloride (B) (450 mg, 2.2 mmol) in THF (8.0 mL) and the amine (A) (455 mg, 2.5 mmol). The reaction mixture was stirred at 70 C for 4 h. After completion, the reaction mixture was partitioned between EtOAc (150 mL) and H2O (100 mL). The org layer was dried (Na2SO4) and concentrated in vacuo to provide the product as a yellow solid. [250 mg, 32%]
[Patent Reference: WO2014149164, page 296, ![]() (23.7 MB)]
(23.7 MB)]