O-Demethylation
(HBr)
Examples:
Example 1
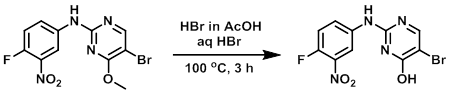
A mixture of the SM (2 g, 5.82 mmol), HBr in AcOH (12 mL), and aq HBr (6 mL) was heated at 100 C for 3 h. The reaction mixture was cooled to RT, extracted with EtOAc (2 x 100 mL), washed with brine, dried (Na2SO4), and concentrated to provide the product as an off-white solid. [1.3 g]
[Patent Reference: WO2010038081, page 125, ![]() (33.8 MB)]
(33.8 MB)]
Example 2
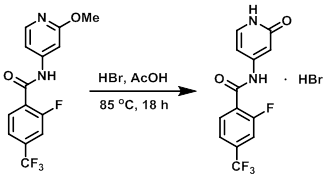
To a 50 L jacketed glass reactor fitted with an N2 inlet and a mechanical stirrer was added AcOH (17.50 L, 7.0 vol) and the SM (2.500 kg, 7.956 mol). The mixture was stirred. A solution of HBr in AcOH (5.853 kg, 3.928 L of 33% w/w, 23.87 mol) was added, resulting in a mild exotherm and a light amber solution. The solution became a darker amber color as more HBr was added. The temperature of the reaction mixture was increased to a mild reflux (70 C internal), over ~30 min, resulting in the generation of a substantial amount of gas (MeBr, HBr). The internal temperature of the reaction mixture was then increased to 85 C over 90 min, and stirring was continued overnight at 85 C. After 16 h, HPLC indicated a complete reaction (less than 1% SM remaining relative to product). The internal temperature of the reaction mixture was reduced from 85 C to 50 C over 30 min, after which time was added toluene (7.500 L, 3.0 vol). Stirring was continued for 10-15 min. The internal temperature of the reaction mixture was then reduced to 20 C, and the mixture was stirred for 1-2 h. The reaction was then filtered, and the wet filter cake was washed with toluene (7.500 L, 3.0 vol) and pulled dry. The solid material was scooped out of the filter and dried in vacuo (40 C, 10-25 mbar, rotovap) to provide the product as a white, crystalline solid. [2.609 kg, 6.846 mol, 86%]
[Patent Reference: WO2015089361, page 76, ![]() (5.2 MB)]
(5.2 MB)]
Example 3
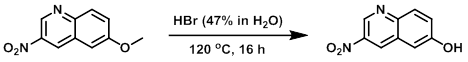
A mixture of the SM (100 mg, 0.49 mmol) in HBr (47% in H2O, 2.5 mL) was stirred at 120 C for 16 h. Upon completion, the reaction mixture was cooled to RT and neutralized with 6N NaOH. The mixture was extracted with EtOAc (150 mL), dried (Na2SO4), concentrated, and purified by silica gel flash chromatography (40-50% EtOAc/hexane) to provide the product. [73 mg, 78%]
[Patent Reference: WO2007084786, page 115, ![]() (9.4 MB)]
(9.4 MB)]
Example 4
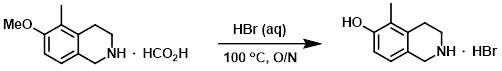
The SM (150 g, 847 mmol) was suspended in aq HBr (48%, 1.0 L) and heated at 100 C overnight. The solvent was removed in vacuo to provide the product which was taken on to the next step without further purification. [195 g]
[Patent Reference: WO2016014463, page 91, ![]() (6.7 MB)]
(6.7 MB)]
