Grignard
(RMgX + Ketone)
Examples:
Example 1
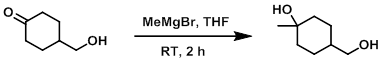
To a solution of the SM (1.0 g, 7.8 mmol) in THF (20 mL) at 0 C was added dropwise MeMgBr (3.0 M in ether, 7.8 mL, 23.4 mmol) over 5 min. The reaction mixture was allowed to warm to RT and stir for 2 h. The mixture was quenched with sat aq NH4Cl and extracted with EtOAc (2 x 20 mL). The combined organics were washed with H2O (20 mL), brine (20 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (80% EtOAc/hexane) to provide the product as a white solid. [300 mg, 27%]
[Patent Reference: WO2015129926, page 98, ![]() (21.5 MB)]
(21.5 MB)]
Example 2
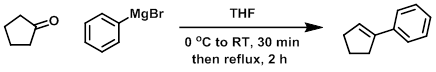
To a solution of 3.0 M phenylmagnesium bromide in ether (49.7 mL, 149 mmol) was added THF (300 mL). The solution was cooled to 0 C and treated with cyclopentanone (13.23 mL, 149 mmol). The reaction was stirred at RT for 30 min, then at reflux for 2 h. Ice (20 g) was added, followed by 6 N HCl, until the precipitate dissolved. The product was extracted with ether. The combined organics were washed with sat aq NaHCO3, dried (MgSO4), concentrated, and purified by silica gel column chromatography to provide the product as a colorless oil. [21.49 g, 100%]
[Patent Reference: WO2011014535, page 50, ![]() (17.3 MB)]
(17.3 MB)]
Example 3
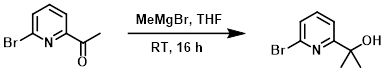
To a solution of the SM (4.0 g, 20 mmol) in anhydrous THF (50 mL) at 0 C was added a solution of MeMgBr (3.0 M, 8.0 mL, 24 mmol) in THF dropwise over 20 min. The reaction mixture was stirred at RT for 16 h. The mixture was quenched with H2O (30 mL) and extracted with EtOAc (4 x 30 mL). The org layer was washed with brine (30 mL), dried (Na2SO4), and concentrated to provide the product which was taken forward without further purification. [4.2 g, 98%]
[Patent Reference: WO2016011390, page 209, ![]() (20.2 MB)]
(20.2 MB)]
Example 4
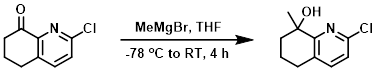
To a solution of the SM (630 mg, 3.5 mmol) in THF (10 mL) at -78 C was slowly added MeMgBr (2M in hexane, 2.6 mL, 5.3 mmol). The reaction mixture was stirred at RT for 4 h, after which time it was quenched with aq NH4Cl. The mixture was extracted with EtOAc (3 x 20 mL). The combined organics were dried and concentrated. The residue was purified by silica gel column chromatography (10:1 PE/EtOAc) to provide the product as a colorless oil. [300 mg, 44%]
[Patent Reference: WO2016011390, page 356, ![]() (20.2 MB)]
(20.2 MB)]
Example 5
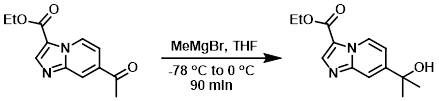
To a suspension of the SM (80 mg, 0.298 mmol) in THF (5 mL) was added MeMgBr (3M in ether, 0.218 mL, 0.655 mmol) at -78 C. The reaction mixture was stirred under N2 at -78 C for 1 h, then 0 C for 30 min. MeOH (0.5 mL) was added to quench the reaction. The solvent was removed in vacuo and the crude product was purified by normal phase chromatography to provide the product as a white solid. [16 mg, 22%]
[Patent Reference: WO2016010950, page 253, ![]() (18.8 MB)]
(18.8 MB)]
