Ester to Acid
(NaOH + H2O/THF)
Examples:
Example 1

A mixture of the SM (36.8 g, 0.12 mol), aq 2N NaOH (850 mL) and dioxane (170 mL) were stirred at 50 C for 24 h. The reaction mixture was cooled to RT, diluted with EtOAc (1.0 L), and acidified to pH 1 using aq 4N HCl. The resulting precipitate was filtered, washed with H2O, and dried in vacuo to provide the product as an off-white solid. [25.4 g]
[Patent Reference: WO2010035166, page 68, ![]() (3.2 MB)]
(3.2 MB)]
Example 2

A 5 mL microwave vial was charged with the SM (16 mg, 0.028 mmol), dioxane (1 mL), and 1N NaOH (0.5 mL). The mixture was irradiated in a microwave reactor at 100 C for 10 min, after which time it was concentrated and purified by reverse phase HPLC (20-95% 0.1% TFA/ACN in 0.1% TFA/H2O) to provide the product as a yellow amorphous solid. [7.6 mg, 67%]
[Patent Reference: WO2012129338, page 151, ![]() (12.0 MB)]
(12.0 MB)]
Example 3
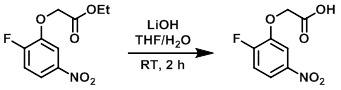
To a solution of the SM (2.0 g, 8.2 mmol) in 1:1 THF/H2O (20 mL) was added LiOH (1.68 g, 70 mmol). The reaction was stirred at RT for 2 h. After completion, the reaction mixture was neutralized with 1M aq citric acid (50 mL) and extracted with EtOAc (50 mL). The org layer was dried (Na2SO4) and concentrated in vacuo. The resulting material was purified by silica gel column chromatography to provide the product as a white solid. [1.4 g, 79.5%]
[Patent Reference: WO2014149164, page 337, ![]() (23.7 MB)]
(23.7 MB)]
Example 4
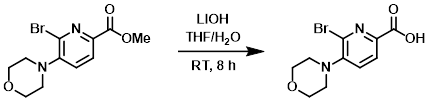
To a solution of the SM (23.0 g, 69.6 mmol) in THF (200 mL) was added LiOH (9.62 g, 229.1 mmol) in H2O (100 mL). The reaction mixture was stirred at RT for 8 h. The mixture was concentrated and the resulting aq solution was adjusted to pH < 4 with 1M HCl. The mixture was extracted with DCM (3 x 100 mL), dried (Na2SO4), and concentrated to provide the product as a yellow solid. [21.0 g, 96%]
[Patent Reference: WO2015140133, page 124, ![]() (11.7 MB)]
(11.7 MB)]
Example 5
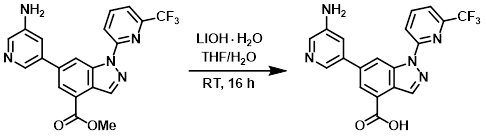
A mixture of the SM (100 mg, 0.24 mmol) and LiOH-H2O (30 mg, 0.72 mmol) in 4:1 THF/H2O (5 mL) was stirred at RT for 16 h. The mixture was adjusted to pH = 6 using 1N HCl and extracted with DCM (2 x 100 mL). The combined organics were washed with H2O (20 mL), concentrated, and purified by Prep HPLC (5-95%, MeOH/H2O as mobile phase) to provide the product as a white solid. [67 mg, 70%]
[Patent Reference: WO2016011390, page 101, ![]() (20.2 MB)]
(20.2 MB)]
Example 6
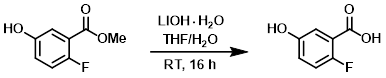
To a solution of the SM (30.0 g, 176.5 mmol) in 3:1 THF/H2O (350 mL) was added LiOH-H2O (22.2 g, 529.4 mmol) at RT. The reaction mixture was stirred at RT for 16 h. The THF was removed from the reaction mixture. The mixture was diluted with cold H2O and the pH was adjusted to about 3 with aq 1.5N HCl. The mixture was extracted with EtOAc (3 x 250 mL). The combined organics were washed with brine (150 mL), dried (Na2SO4), and concentrated to provide the product as an off white solid. [25.1 g, 91%]
[Patent Reference: WO2015051043, page 62, ![]() (9.7 MB)]
(9.7 MB)]
