Bromination
Examples:
Example 1
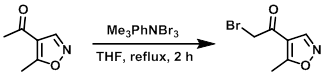
To the SM (340 mg, 2.7 mmol) in dry THF at RT was added Me3PhNBr3 (1.12 g, 3 mmol). The mixture was refluxed for 2 h, after which time it was quenched with H2O (2 x 20 mL) and extracted with EtOAc. The organic layer was dried (Na2SO4), concentrated, and purified by flash chromatography (15% EtOAc/hexane) to provide the product. [100 mg] [WO2010038081]
Example 2
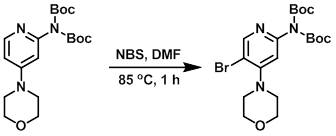
The SM (51 mg, 0.29 mmol) was dissolved in DMF (20 mL). NBS (51.6 mg, 0.29 mmol) was added and the mixture was heated to 85 C for 1 h. The mixture was concentrated and purified by flash chromatography (0-40% EtOAc/hexane) to provide the product. [95 mg] [WO2010038081, page 276]
Example 3
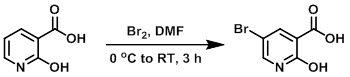
To a solution of the SM (5.0 g, 35.94 mmol) in dry DMF at 0 C was added a solution of Br2 (9.19 g, 57.51 mmol) in cooled DMF dropwise over 1 h. The reaction mixture was stirred at RT for 3 h, after which time it was quenched with ice-H2O. The resulting yellow solid was filtered, washed with H2O, and dried under vacuum to provide the product. [6 g] [WO2010038081, page 238]
Example 4
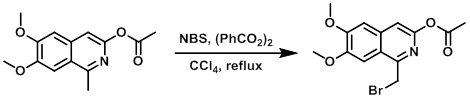
To a solution of the SM (261 mg, 1.00 mmol) in dry CCl4 (5 mL) in a 10 mL microwave vial was added benzoyl peroxide (12 mg, 0.05 mmol), followed by NBS (178 mg, 1.00 mmol). The resulting mixture was irradiated in a microwave reactor at 130 C for 15 min. The reaction mixture was diluted with DCM (20 mL), washed with brine (5 mL), dried (Na2SO4), concentrated, and purified by silica gel chromatography (0-25% EtOAc/cyclohexane) to provide the product as a yellow solid. [140 mg, 41%] [WO2012112946, page 165]
Example 5

To a solution of the SM (603 mg, 2.75 mmol) in AcOH (27 mL) was added dropwise Br2 (0.15 mL, 2.92 mmol). The resulting mixture was stirred at RT 2 h. The resulting solids were filtered and washed with AcOH (20 mL), EtOAc (30 mL), Et2O (4 x 7 mL), DCM (55 mL), sat aq NaHCO3 (15 mL), and finally H2O (10 mL) to provide the product as a yellow solid. [718 mg] [WO2012112946, page 216]
