Amine to Amide
(T3P)
Examples:
Example 1

To a solution of the SM (2.92 g, 9.89 mmol) in 2-methyl tetrahydrofuran (115 mL) was added DIEA (13.8 mL, 39.5 mmol) and cyclopropylamine (2.77 mL, 39.5 mmol). The resulting mixture was heated to 70 C and T3P (50% weight solution in DCE) (17.3 mL, 59.3 mmol) was added. The reaction mixture was heated at 70 C for 16 h and then diluted with EtOAc (200 mL). The mixture was then washed sequentially with sat aq Na2CO3 (100 mL), 10% aq citric acid (100 mL), and saturated aq Na2CO3 (100 mL). The combined org extracts were dried (MgSO4) and concentrated in vacuo to give an oil. The crude was purified by column chromatography (eluting with 50% EtOAc/heptane) to give a solid which was recrystallized with IPA to provide the product as a white solid. [2.46 g, 74%]
[Patent Reference: WO2010032200, page 94, ![]() (6.2 MB)]
(6.2 MB)]
Example 2
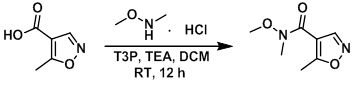
To a mixture of the acid (500 mg, 3.5 mmol), TEA (1.19 g, 11.8 mmol) and the amine (498 mg, 5.1 mmol) in DCM was slowly added T3P (50% in EtOAc, 3.3 mL, 6 mmol) at 0 C. The reaction mixture was slowly allowed to warm to RT and stirred for 12 h. The reaction was diluted with DCM (12 mL) and the mixture was washed successively with H2O (2 x 50 mL), 10% aq NaHCO3 (50 mL), and brine. The org layer was dried (Na2SO4), concentrated, and purified by column chromatography (15% EtOAc/hexane) to provide the product. [480 mg]
[Patent Reference: WO2010038081, page 134, ![]() (33.8 MB)]
(33.8 MB)]
Example 3
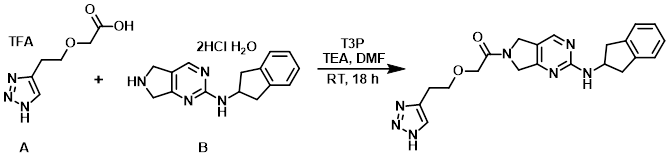
To a stirring solution of the acid (A) (20.22 g, 70.90 mmol), the amine (B) (27.99 g, 81.54 mmol), and TEA (98.83 mL, 709.03 mmol) in DMF (404.4 mL) at 0 C was added a solution of T3P (50% solution in DMF, 51.89 mL, 81.54 mmol) over 30 min. The reaction mixture was stirred at RT for 18 h. The mixture was concentrated in vacuo and the resulting residue was diluted with H2O (200 mL) and extracted with EtOAc (4 x 250 mL) and DCM (4 x 250 mL). The combined organics were washed with sat aq NaHCO3 (2 x 100 mL), brine (100 mL), dried (Na2SO4), and concentrated to give a red solid (25.70 g). The material was slurried in 10% MeOH/EtOAc (200 mL) for 2 h at RT, filtered, and the solids were washed with cold EtOAc (50 mL) to give a solid (18.2 g) which was re-slurried in EtOAc (200 mL) at reflux for 1 h. The mixture was cooled to RT, stirred 1 h, and filtered to give a light pink solid. The material was slurried in 1:1 MeOH/H2O (200 mL) and stirred at 50 C for 30 min, then NH4OH (32%, 50 mL) was added and stirring was continued at 50 C for 30 min. Upon cooling to RT, additional NH4OH (32%, 50 mL) was added and stirring was continued at RT for 1 h. The resulting light gray solid was filtered, dried, and slurried again in EtOAc (200 mL) for 1 h. The solids were filtered, washed with EtOAc (25 mL), and dried to provide the product as a gray solid. [12.42 g, 43%]
[Patent Reference: WO2014110000, page 21, ![]() (2.6 MB)]
(2.6 MB)]
Example 4

A 50 L jacketed glass reactor was fitted with an N2 inlet, a mechanical stirrer, and a condenser. The stirrer was set to 150 rpm and the jacket temperature set at 40 C. To the reactor was added 2-Me-THF (6.000 L, 3.0 vol), the acid (2.0 kg, 9.610 mol), the amine (1.278 kg, 10.09 mol), and TEA (2.917 kg, 4.018 L, 28.83 mol), resulting in a slightly hazy, light amber solution. The reactor was switched to reaction control and heated to 35 C. To the solution was added T3P in 2-Me-THF (9.176 kg, 9.176 L of 50% w/w, 14.42 mol) over 30-45 min, resulting in a light amber solution. After 2 h, the reaction was judged to be complete by HPLC analysis. The reaction was quenched with H2O (1.000 L, 0.5 vol), which was added via addition funnel over 10 min to control the exothermic quenching reaction. The mixture was diluted with 2-Me-THF (8.000 L, 4.0 vol) and H2O (8.000 L, 4.0 vol), and stirred for 30 min at 30-40 C. After the stirring was stopped, the layers were allowed to separate, and the aq layer was removed. The organic layer was washed with 10% aq NaOH (6.00 L, 3.0 vol). After stirring, an emulsion resulted. Brine (500 mL, 0.25 vol) was added and the mixture was stirred 5 min, after which time layers separated, and the aq layer was removed. The org layer was washed again with brine (10.00 L, 5.0 vol), and the aq layer was removed. The org layer was dried (Na2SO4), then filtered through celite. The filter cake was washed with 2-Me-THF (4.000 L, 2.0 vol), and pulled dry. The filtrate was transferred to a rotovap, and partial distillation of solvent was begun (40 C, 150 mbar), resulting in the formation of solids in the mixture. Cyclohexane (10.00 L, 5.0 vol) was added portionwise during the partial distillation. Distillation was stopped, the mixture (~8 L) was slurried on the rotovap, and the bath temp was reduced to RT. The mixture was filtered, and the filter cake was washed with cyclohexane (2.000 L, 1.0 vol), and pulled dry under a N2 blanket to afford a light yellow solid. The solid was scooped out of the funnel and dried in vacuo (40 C, <30 mbar, rotovap) to provide the product as a fine off-white solid. [2.501 kg, 7.959 mol, 83%]
[Patent Reference: WO2015089361, page 75, ![]() (5.2 MB)]
(5.2 MB)]
