Alkene to Alkane
![]()
Common Conditions:
H2 + Pd/C
Catalytic hydrogenation with palladium on carbon (Pd/C) is a very common method for reducing alkenes. Typical solvents include MeOH, EtOH, and EtOAc.[1][2]
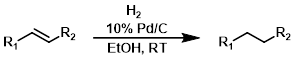
H2 + PtO2
Catalytic hydrogenation with platinum(IV) oxide (PtO2) effectively reduces alkenes. PtO2 is often used in place of Pd/C for substrates where dehalogenation of aromatic halides is a concern.[2][3]
![]()
HCO2NH4 + Pd/C
Catalytic transfer hydrogenation using ammonium formate and palladium on carbon (Pd/C) is a common method for alkene reduction. Ammonium formate is easier to handle than hydrogen gas and measuring precise equivalents is simpler.
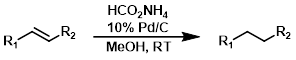
Zn
Zinc is generally not capable of reducing isolated double bonds. Conjugation of a double bond with an EWG makes reduction with zinc possible. The process is useful in cases where the EWG (ex. benzylic ketone) is susceptible to reduction under hydrogenation conditions.[2][4]
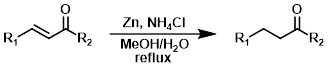
NaHB(OAc)3
Sodium triacetoxyborohydride (STAB) is a useful reagent for the reduction of enamines. Other reagents can effect the same reduction, but STAB is a good choice when mild conditions with high functional group tolerance are required.[2][5]
3_2.png)
LiAlH4
Lithium aluminum hydride (LiAlH4) doesn't normally reduce alkenes, but alkenes that are conjugated to nitro groups (nitroalkenes) are often reduced with LiAlH4. The nitro group is reduced as well. This is a common procedure after nitroalkenes have been formed using Henry reactions.[4]
![]()
References:
1) Carey, F. A.; Sundberg, R. J.; Advanced Organic Chemistry, Part B: Reactions and Synthesis, 5th Edition
2) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
3) Augustine, R. L.; Heterogeneous Catalysis for the Synthetic Chemist, 1st Edition
4) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
5) Caron, S.; Practical Synthetic Organic Chemistry
