Alcohol to Chloride
![]()
Common Conditions:
SOCl2 (neat)
Alcohol conversion to a chloride in neat thionyl chloride (SOCl2) is typically done at reflux (76 C).
![]()
SOCl2 + Solvent
When thionyl chloride (SOCl2) chlorinations are done with a solvent then DCM is the usual choice.
![]()
SOCl2 + Solvent + DMF (cat.)
Adding DMF as a catalyst leads to the formation of dimethylchloromethyleneammonium chloride (Vilsmeier-Haack reagent), which likely acts as the active chlorinating agent.[1]
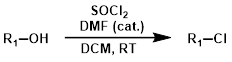
Sulfonyl Chlorides (R-SO2Cl)
Sulfonyl chlorides such as methanesulfonyl chloride (MsCl) and 4-toluenesulfonyl chloride (TsCl) offer milder, non-acidic reaction conditions.[2]
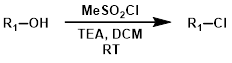
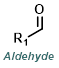
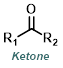
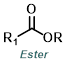
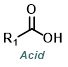
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||||
 |
 |
|||
 |
 |
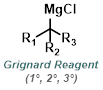 |
||
 |
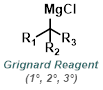 |
|||
 |
 |
|||
 |
References:
1) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups
2) Kim, H.; Lee, Y.; Shetty, D.; Lee, H.; Lee, D.; Chung, J.; Lee, M.; Chung, K.; Jeong, J; Bull. Korean Chem. Soc. 2010, Vol 31, No. 11 (Link)
