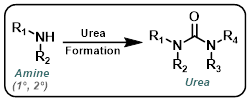
Urea Formation
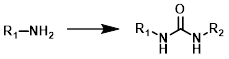
Common Conditions:
Amine + Isocyanate
When one of the reagents is available as the corresponding isocyanate it provides a very simple method for the formation of ureas. The reaction is generally done in a suitable solvent (ex. DMF, THF, or DCM) at RT. No base is required.
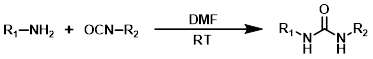
Amine + Carbamate
The reaction of amines with reactive carbamates provides urea products. The most useful carbamates are isopropenyl carbamates because they react irreversibly. Phenyl carbamates are often used, but their reactions are more prone to reversibility and side-pdt formation.[1]
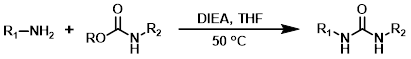
CDI
Carbonyldiimidazole (CDI) can be a useful alternative to highly toxic phosgene-related reagents. The reagent order of addition can be important for avoiding the formation of symmetrical urea by-pdts.[2]
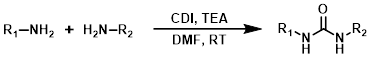
Triphosgene
Triphosgene is solid reagent that serves as a easier to handle substitute for phosgene (gas). During reactions triphosgene forms phosgene, which is highly toxic. The reagent order of addition can be important for avoiding the formation of symmetrical urea by-pdts.[3]

Curtius Rearrangement
The Curtius Rearrangement reaction can be useful when one of the reagents is a carboxylic acid, rather than an amine. To control N2 evolution, large-scale reactions are typically run with dose-controlled addition of DPPA at a moderate temperature.[4]

Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
||
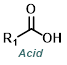 |
||
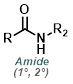 |
||
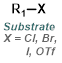 |
References:
1) Gallou; I. et al. J. Org. Chem., 2005, 70(17), 6960-6963
2) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups
3) Wikipedia: Triphosgene (link)
4) Anderson, N. G.; Practical Process Research and Development, a Guide for Organic Chemists, 2nd Edition
