Substitution (I)
(N-Heteroaryls)
Examples:
Example 1
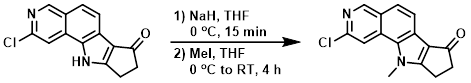
To a suspension of the SM (52 mg, 0.2 mmol) in THF (4 mL) was added NaH (60%, 16 mg, 0.4 mmol) at 0 C, and the mixture was stirred for 15 min. To this mixture was added MeI (42 mg, 0.3 mmol) in THF (1 mL) dropwise at 0 C. The reaction mixture was warmed to RT and stirred for 4 h. The mixture was quenched with aq Na2CO3, extracted with EtOAc (3x), and washed with brine. Column purification (20-100% EtOAc/heptane) provided the product as an off-white powder. [31 mg, 60%]
[Patent Reference: WO2014149164, page 415, ![]() (23.7 MB)]
(23.7 MB)]
Example 2
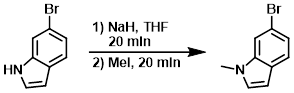
A 1L RBF was charged with the SM (14.9 g, 76.0 mmol) and THF (150 mL) to give a dark maroon solution. The flask was put under N2, and NaH (60% wt, 3.64 g, 91.0 mmol) was added in several portions over 15 min. The resulting mixture was stirred for 20 min, then MeI (5.94 mL, 95.0 mmol) was added via syringe. Within a few minutes and exotherm was observed. After stirring for 20 min, the mixture was concentrated in vacuo, and the residue was taken up in sat aq NaHCO3 and extracted with DCM (3x). The combined organics were dried (Na2SO4), concentrated, and purified by silica gel chromatography (0-40% EtOAc/heptane) to provide the product as a lightly colored oil. [14.4 g, 91%]
[Patent Reference: WO2014201173, page 233, ![]() (19.7 MB)]
(19.7 MB)]
Example 3
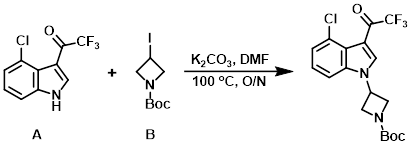
A mixture of the indole (A) (8.00 g, 32.31 mmol), the alkyl iodide (B) (10.06 g, 35.54 mmol), and K2CO3 (13.40 g, 96.93 mmol) in DMF (30 mL) was stirred at 100 C overnight. After cooling to RT, the mixture was quenched with H2O (50 mL) and extracted with EtOAc (3 x 80 mL). The combined organics were dried (Na2SO4) and concentrated in vacuo. The residue was purified by silica gel column chromatography (1:5 EtOAc/PE) to provide the product as a yellow solid. [9.60 g, 80%]
[Patent Reference: WO2016100281, page 210, ![]() (10.3 MB)]
(10.3 MB)]
Example 4
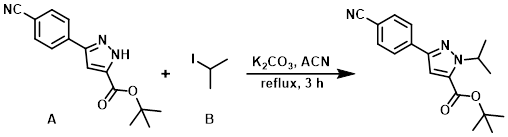
To a solution of the pyrazole (A) (15.7 g, 58.3 mmol) in ACN (200 mL) was added K2CO3 (16.1 g, 117 mmol) and the alkyl iodide (B) (8.73 mL, 87.4 mmol). The resulting mixture was stirred at reflux for 3 h. The reaction mixture was then partitioned between EtOAc (300 mL) and H2O (300 mL). The aq layer was further extracted with EtOAc (2 x 200 mL) and the combined organics were washed with brine (200 mL), dried (MgSO4), and concentrated in vacuo to provide an off-white solid. The crude was purified by silica gel column chromatography (eluting with 20% EtOAc/pentane) to provide the product as a white solid. [16.3 g, 90%]
[Patent Reference: WO2010032200, page 164, ![]() (6.2 MB)]
(6.2 MB)]
Example 5
![]()
A solution of the SM (1.0 g, 14.5 mmol), MeI (3.1 g, 21.7 mmol), and K2CO3 (4.0 g, 28.9 mmol) in THF (15 mL) was stirred at RT for 3 h. EtOAc (20 mL) and H2O (10 mL) were added and the layers were separated. The org layer was concentrated and the resulting residue was purified by silica gel chromatography (eluting with 10% MeOH/DCM) to provide the product as a yellow oil. [860 mg, 71%]
[Patent Reference: WO2015140133, page 115, ![]() (11.7 MB)]
(11.7 MB)]
Example 6
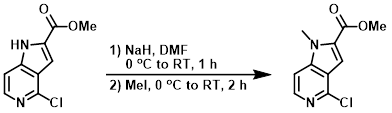
To a 0 C solution of the SM (0.5 g, 2.36 mmol) in anhydrous DMF (3 mL) was added NaH (60% in mineral oil, 0.113 g, 2.84 mmol), and the mixture was stirred at RT for 1 h. The reaction mixture was cooled again to 0 C and treated with MeI (0.402 g, 2.83 mmol), and stirring was continued for 1 h. The mixture was allowed to warm to RT and stir for another 1 h. The mixture was diluted with H2O and the solids were filtered and dried under vacuum to provide the product as a yellow solid. [0.460 g, 86%]
[Patent Reference: WO2015088045, page 134, ![]() (10.3 MB)]
(10.3 MB)]
Example 7
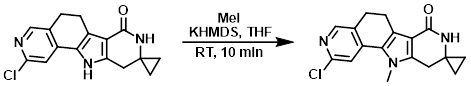
To a solution of the SM (30 mg, 0.1 mmol) in THF (2.0 mL) at 0 C was slowly added KHMDS (25 mg, 0.1 mmol). The mixture was stirred for 10 min, after which time MeI (14 mg, 0.1 mmol) was added slowly over 10 min. The reaction was stirred at RT for 10 min. Upon completion, the reaction mixture was quenched with aq NH4Cl (10 mL). The mixture was extracted with EtOAc (20 mL), dried (Na2SO4), and concentrated in vacuo. The resulting material was purified by prep TLC to provide the product as a pale brown solid. [20.0 mg, 64.5%]
[Patent Reference: WO2014149164, page 229, ![]() (23.7 MB)]
(23.7 MB)]
