Substitution (Cl)
(Cyanide)
Examples:
Example 1
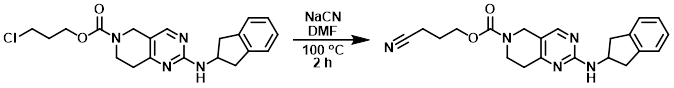
A solution of the SM (0.42 g, 1.09 mmol) and NaCN (0.085 g, 1.73 mmol) in DMF (3 mL) was stirred at 100 C for 2 h. The reaction mixture was cooled to RT, diluted with DCM (20 mL), and washed with H2O (25 mL). The aq layer was further extracted with DCM (2 x 20 mL). The combined organics were dried (Na2SO4), concentrated, and purified by column chromatography (0-10% MeOH/DCM) to provide the product as a light brown oil which was used in the next step without further purification.
[Patent Reference: WO2014110000, page 35, ![]() (2.6 MB)]
(2.6 MB)]
Example 2

A mixture of the SM (298.0 g, 1.8 mol) and NaCN (154.5 g, 2.1 mol) in DMF (1.2 L) was stirred at RT for 12 h. The mixture was partitioned between H2O and EtOAc. The layers were separated and the org layer was dried (Na2SO4) and concentrated. The residue was purified by silica gel chromatography (1:50 EtOAc/PE) to provide the product. [230.0 g, 79%]
[Patent Reference: WO2016014463, page 91, ![]() (6.7 MB)]
(6.7 MB)]
