Ketone to Alcohol
(NaBH4)
Examples:
Example 1
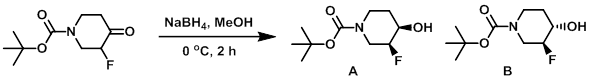
To a solution of the SM (15.5 g, 71.3 mmol) in MeOH (150 mL) at 0 C was added NaBH4 (3.51 g, 93.7 mmol). The resulting mixture was stirred at 0 C for 2 h, after which time it was allowed to warm to RT. Sat aq NH4Cl (200 mL) was added and the mixture was extracted with EtOAc. The combined organics were washed with brine, dried (MgSO4), and concentrated. The resulting material was purified by silica gel column chromatography (eluting with 40-50% EtOAc/heptane) to provide product B as a pale yellow oil which solidified on standing to a white solid (3.81 g, 24%). The second eluting compound was product A, which was isolated as a white solid (10.57 g, 68%). [combined yield: 14.38 g, 92%]
[Patent Reference: WO2012069948, page 82, ![]() (3.9 MB)]
(3.9 MB)]
Example 2
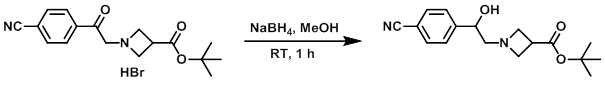
To a mixture of the SM (170 mg, 0.446 mmol) in MeOH (5 mL) was added NaBH4 (25 mg, 0.661 mmol). The reaction mixture was stirred at RT for 1 h, after which time it was quenched with H2O. The mixture was diluted with EtOAc. The layers were separated and the org layer was dried (MgSO4) and concentrated to provide the product. [100 mg]
[Patent Reference: WO2011017578, page 127, ![]() (8.4 MB)]
(8.4 MB)]
Example 3
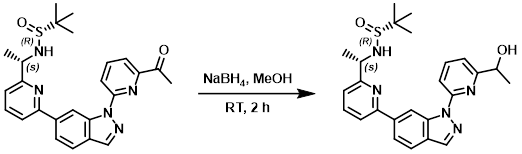
To a solution of the SM (2.0 g, 4.33 mmol) in MeOH (30 mL) at RT was added NaBH4 (320 mg, 8.66 mmol). The reaction mixture was stirred at RT for 2 h. After concentration, the residue was dissolved in DCM (50 mL) and washed with H2O (15 mL). The org layer was washed with brine, dried (Na2SO4), and concentrated to provide the product which was used without further purification. [1.23 g, 85%]
[Patent Reference: WO2016011390, page 351, ![]() (20.2 MB)]
(20.2 MB)]
