Cyanation
Examples:
Example 1
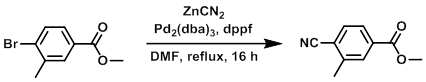
To a solution of the SM (16.53 g, 72 mmol) in DMF (20 mL) was added dppf (8 g, 14.43 mmol) and ZnCN2 (5.08 g, 43.3 mmol), followed by Pd2(dba)3 (6.61 g, 7.22 mmol). The mixture was stirred at reflux for 16 h, cooled to RT, and concentrated in vacuo. EtOAc (1.0 L) was added to the crude and the resulting suspension was filtered. The filtrate was washed with H2O (1.0 L) and the aq layer extracted with EtOAc (2 x 1.0 L). The combined org extracts were washed with brine (1.0 L), dried (MgSO4), and concentrated in vacuo. The resulting material was purified by column chromatography (eluting with 5% EtOAc/pentane) to provide the product as a solid. [13.7 g, 88%]
[Patent Reference: WO2010032200, page 132, ![]() (6.2 MB)]
(6.2 MB)]
Example 2
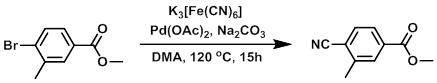
To a solution of the SM (4.0 g, 17.46 mmol) in DMA (10 mL) was added K3[Fe(CN)6] (3.70 g, 17.5 mmol), followed by Na2CO3 (1.85 g, 17.5 mmol), then Pd(OAc)2 (78 mg, 0.35 mmol). The mixture was heated at 120 C for 15 h, after which time it was quenched onto H2O (150 mL) and extracted with MTBE (3 x 50 mL). The combined org extracts were washed with H2O (150 mL), dried (MgSO4), and concentrated in vacuo to provide the product as a solid. [2.53 g, 83%]
[Patent Reference: WO2010032200, page 133, ![]() (6.2 MB)]
(6.2 MB)]
Example 3
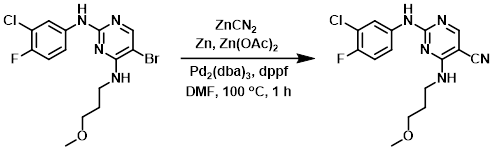
A stirred suspension of the SM (4.01 g, 10.3 mmol), zinc powder (168 mg, 2.57 mmol), zinc cyanide (784 mg, 6.67 mmol), Pd2(dba)3 (188 mg, 0.210 mmol), dppf (230 mg, 0.410 mmol), and zinc acetate (75 mg, 0.41 mmol) in degassed DMF (25 mL) was prepared. The vessel was purged with N2 for 1 min. The mixture was placed under N2 and heated to 100 C for 1 h. The reaction mixture was allowed to cool to RT, after which time it was diluted with H2O until maximum precipitation was acheived. The resulting suspension was stirred for 10 min, filtered, and the filter cake washed with H2O. The crude was purified by normal phase chromatography (0-3% MeOH/DCM) to provide the product. [3.3 g, 95%]
[Patent Reference: WO2010038081, page 113, ![]() (33.8 MB)]
(33.8 MB)]