Bromination
(Alpha Bromination)
Examples:
Example 1
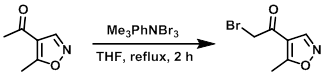
To the SM (340 mg, 2.7 mmol) in dry THF at RT was added Me3PhNBr3 (1.12 g, 3 mmol). The mixture was refluxed for 2 h, after which time it was quenched with H2O (2 x 20 mL) and extracted with EtOAc. The organic layer was dried (Na2SO4), concentrated, and purified by silica gel column chromatography (15% EtOAc/hexane) to provide the product. [100 mg]
[Patent Reference: WO2010038081, page 135, ![]() (33.8 MB)]
(33.8 MB)]
Example 2
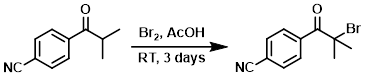
To a mixture of the SM (2.4 g, 13.86 mmol) in AcOH (50 mL) was added Br2 (0.80 mL, 15.53 mmol). The mixture was stirred at RT for 3 days, after which time it was concentrated in vacuo. The resulting material was diluted with EtOAc, washed with sat NaHCO3, dried (MgSO4), and concentrated. The residue was purified by silica gel chromatography (EtOAc/hexane) to provide the product. [2.5 g]
[Patent Reference: WO2011017578, page 154, ![]() (8.4 MB)]
(8.4 MB)]
Example 3
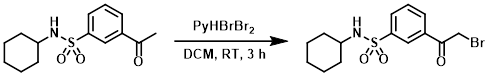
To a solution of the SM (2.98 g, 10.6 mmol) in dry DCM was added in portions pyridinium tribromide (3.765 g, 11.8 mmol). The reaction was stirred at RT for 3 h, after which time the reaction mixture was partitioned between EtOAc and brine. The layers were separated and the aq layer was further extracted with EtOAc. The combined organics were washed with brine, dried (MgSO4), concentrated, and purified by column chromatography to provide the product as a colorless oil which crystallized to a white solid upon standing. [0.893 g, 23%] [UK Pat App GB2463151A, page 220]
Example 4
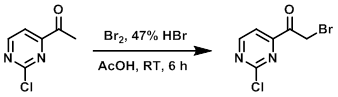
To a solution of the SM (1.0 g, 6.4 mmol) in AcOH (15 mL) was added Br2 (0.3 mL, 6.4 mmol) and 47% HBr (0.7 mL, 6.4 mmol). The resulting mixture was stirred at RT for 6 h. After completion, the mixture was concentrated in vacuo and the resulting material was washed with ether to provide the product as a light brown solid. [0.7 g, 46%]
[Patent Reference: WO2014149164, page 339, ![]() (23.7 MB)]
(23.7 MB)]
Example 5
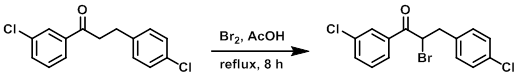
To a solution of the SM (1.39 g, 5 mmol) in AcOH (15 mL) at 0 C was added Br2 (800 mg, 5 mmol) dropwise. The reaction was refluxed for 8 h. The mixture was cooled to RT, concentrated, and diluted with EtOAc (25 mL) and H2O (5 mL). The org layer was washed with brine (5 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by chromatography (50:1 PE/EtOAc) to provide the product as a brown solid. [358 mg, 20%]
[Patent Reference: WO2016023832, page 72, ![]() (3.2 MB)]
(3.2 MB)]
