Benzyl Protection
(Benzyl Bromide)
Examples:
Example 1
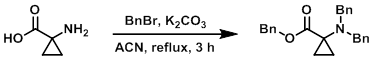
To a solution of the SM (10.0 g, 99.0 mmol) in ACN (300 mL) was added K2CO3 (39.4 g, 285 mmol) and BnBr (41.0 mL, 346.0 mmol). The reaction mixture was stirred at reflux for 3 h. Upon completion, the reaction mixture was quenched with H2O, extracted with EtOAc, dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (10% EtOAc/hexane) to provide the product as a white solid. [30 g, 78%]
[Patent Reference: WO2014149164, page 232, ![]() (23.7 MB)]
(23.7 MB)]
Example 2
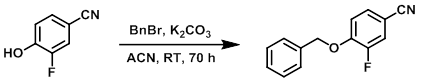
To a stirred solution of the SM (1.00 g, 7.30 mmol) in ACN (20 mL) was added portionwise K2CO3 (2.02 g, 14.6 mmol). The mixture was stirred for 10 min, after which time was added BnBr (1.33 mL, 10.9 mmol).The reaction mixture was stirred at RT for 70 h. The mixture was diluted with EtOAc and H2O. The layers were separated and the org phase was washed with H2O, brine, dried (MgSO4), and concentrated in vacuo. The resulting material was purified by flash chromatography (eluting with 5-20% EtOAc/heptane) to provide the product as a white solid. [1.33 g]
[Patent Reference: WO2012069948, page 57, ![]() (3.9 MB)]
(3.9 MB)]
Example 3
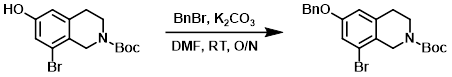
A solution of the SM (22.5 g, 68.6 mmol) and K2CO3 (28.4 g, 205.7 mmol) in DMF (200 mL) was stirred at RT for 2 h. The mixture was then treated with BnBr (11.7 g, 68.6 mmol) and stirred at RT overnight. The reaction mixture was diluted with H2O and extracted with EtOAc. The org layer was dried and concentrated in vacuo. The crude product was purified by silica gel flash chromatography to provide the product. [20 g, 70%]
[Patent Reference: WO2016014463, page 105, ![]() (6.7 MB)]
(6.7 MB)]
