Aryl-Halide to Aryl-OH
[1) Borylation 2) NaBO3]
Examples:
Example 1
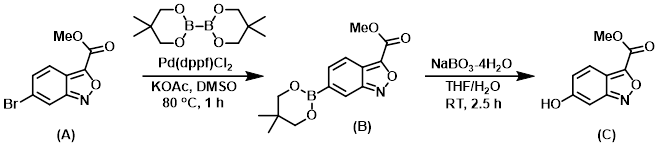
Step 1:
To a sealable vial containing a suspension of KOAc (138 mg, 1.406 mmol), SM (A) (120 mg, 0.469 mmol), and bis(neopentyl glycolato)diboron (138 mg, 0.609 mmol) in DMSO (1 mL), purged with argon for 10 min, was added Pd(dppf)Cl2 (34.3 mg, 0.047 mmol). The vial was capped and the reaction was stirred at 80 C for 1 h. The mixture was diluted with H2O and extracted with EtOAc (2x). The combined organics were concentrated and the residue was purified by flash chromatography (0-100% EtOAc/hexane) to provide Intermediate (B) as a white solid. [65 mg, 48%]
Step 2:
A homogeneous mixture of Intermediate (B) (65 mg, 0.225 mmol) in THF (2 mL) at RT was treated with a mixture of NaBO3-4H2O (41.5 mg, 0.270 mmol) in H2O (2 mL) for 2.5 h. The reaction was quenched with sat aq NH4Cl, then extracted with EtOAc (2x). The combined organics were dried (MgSO4) and concentrated. The residue was purified by flash chromatography (0-100% EtOAc/hexane) to provide the product (C) as a yellow solid. [40 mg, 92%]
[Patent Reference: WO2016010950, page 220, ![]() (18.8 MB)]
(18.8 MB)]
