Amine to Amide
(HBTU)
Examples:
Example 1
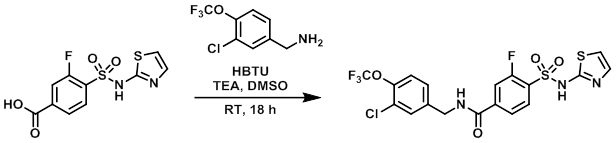
To a suspension of the amine (3.0 g, 11.4 mmol), the acid (2.88 g, 9.54 mmol), and TEA (4.81 mL, 34.5 mmol) in DMSO (30 mL) was added HBTU (4.34 g, 34.5 mmol). The reaction mixture was stirred at RT for 18 h, after which time it was diluted with EtOAc (100 mL), washed with H2O (100 mL), brine (3 x 100 mL), dried (Na2SO4), and concentrated to a brown solid. The resulting material was triturated in MTBE then EtOAc to provide the product. [3.97 g, 7.8 mmol]
[Patent Reference: WO2010035166, page 56, ![]() (3.2 MB)]
(3.2 MB)]
Example 2

To a solution of the acid (250 mg, 0.85 mmol) in 2-methyl tetrahydrofuran (5 mL) was added HBTU (642 mg, 1.69 mmol), followed by pyridine (0.21 mL, 2.54 mmol), and then the amine (0.19 mL, 1.10 mmol). The reaction mixture was stirred at reflux for 1 h and then allowed to stand at RT for 16 h. The mixture was evaporated in vacuo to give a gum. The resulting crude material was partitioned between sat aq NaHCO3 (10 mL) and DCM (5 mL). The org layer was washed with aq 10% citric acid (10 mL) and concentrated in vacuo to provide the product as a solid. [321 mg, 82%]
[Patent Reference: WO2010032200, page 73, ![]() (6.2 MB)]
(6.2 MB)]
Example 3
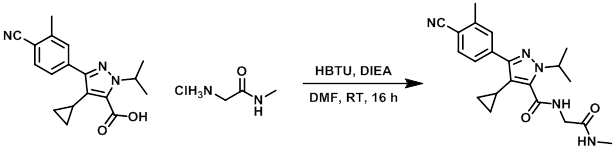
To a solution of the acid (50 mg, 0.16 mmol), the amine (24 mg, 0.19 mmol), and DMF (1 mL) at 5 C was added HBTU (86 mg, 0.19 mmol) followed by DIEA (0.23 mL, 0.65 mmol). The reaction mixture was stirred at RT for 16 h. The reaction mixture was then diluted with EtOAc (25 mL) and washed sequentially with 10% aq citric acid, sat aq NaHCO3 (10 mL), and brine (10 mL). The combined organics were dried (MgSO4) and concentrated in vacuo to give a gum. The resulting crude material was purified by column chromatography (30% DCM/EtOAc) to provide the product as a solid. [42 mg, 68%]
[Patent Reference: WO2010032200, page 121, ![]() (6.2 MB)]
(6.2 MB)]
