Amine to Amide
(Acid Cl + Amine)
Examples:
Example 1
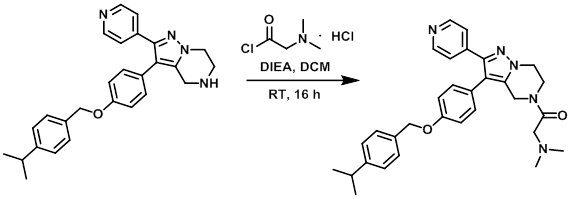
To a stirring solution of the amine (100 mg, 0.236 mmol) in DCM (2 mL) at 0 C under N2 was added DIEA (0.203 mL, 1.18 mmol), followed by the acid chloride (88 mg, 0.471 mmol). The reaction mixture was stirred at RT for 16 h. The mixture was quenched with H2O and extracted with DCM. The org layer was washed with brine, dried (MgSO4), and concentrated in vacuo. The material was purified by Prep LC (12 g silica, 0-12% MeOH/DCM) to provide a white solid (83 mg). The material was purified by Reverse Phase chromatography to provide 55 mg of oil. The oil was diluted with ether and concentrated to provide the product as a waxy solid. [53 mg, 44%]
[Patent Reference: WO2015144799, page 286, ![]() (18.8 MB)]
(18.8 MB)]
Example 2
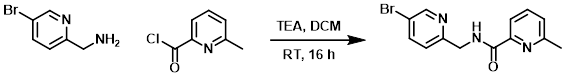
A mixture of the amine (250 mg, 1.34 mmol), the acid chloride (208 mg, 1.34 mmol), and TEA (270 mg, 2.68 mmol) in DCM (10 mL) was stirred at RT for 16 h. After concentration, the residue was purified by silica gel chromatography (8:1 PE/EtOAc) to provide the product as a yellow solid. [300 mg, 73%]
[Patent Reference: WO2016011390, page 90, ![]() (20.2 MB)]
(20.2 MB)]
Example 3
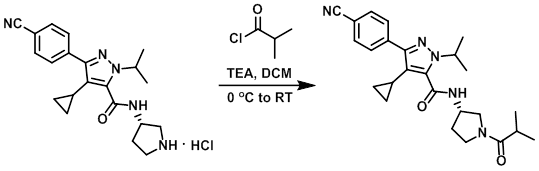
To a solution of the amine (46 mg, 0.12 mmol) and TEA (48 uL, 0.35 mmol) in DCM (2 mL) was added the acid chloride (18 uL, 0.17 mmol). The reaction mixture was stirred at RT for 2 h, after which time it was washed with sat aq NaHCO3 (5 mL) then 10% aq citric acid (5 mL). The org layer was concentrated in vacuo to provide the product as a solid. [40 mg, 80%]
[Patent Reference: WO2010032200, page 76, ![]() (6.2 MB)]
(6.2 MB)]
Example 4
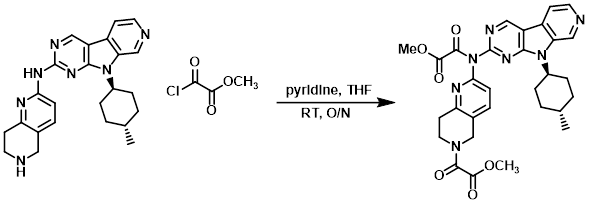
To a solution of the amine (83 mg, 0.2 mmol) in THF (10 mL) at RT was added pyridine (129 uL, 1.6 mmol) and the acid chloride (74 uL, 0.8 mmol). The resulting mixture was stirred at RT overnight, after which time it was concentrated and the resulting material was used in the next step without further purification.
[Patent Reference: WO2012129344, page 140, ![]() (7.3 MB)]
(7.3 MB)]
Example 5
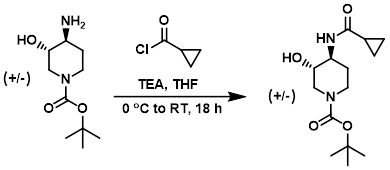
To a solution of the amine (7.52 g, 34.8 mmol) in THF (100 mL) was added TEA (4.85 mL, 34.8 mmol) and the acid chloride (3.18 mL, 35.0 mmol) at 0 C. The reaction was allowed to warm to RT and stir for 18 h, after which time it was concentrated in vacuo and the crude material was diluted with EtOAc. Insoluble material was removed by filtration and the solids were washed with EtOAc (3 x 50 mL). The combined filtrates were concentrated in vacuo and the resulting material was purified by silica gel chromatography (0-5% MeOH/EtOAc) to provide the product as a solid. [1.30 g, 13%]
[Patent Reference: WO2014177977, page 75, ![]() (6.0 MB)]
(6.0 MB)]
Example 6
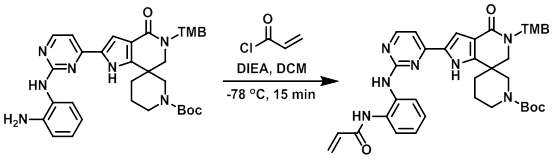
To a solution of the amine (65 mg, 0.1 mmol) in DCM (0.5 mL) was added DIEA (25 mg, 0.2 mmol). The mixture was cooled to -78 C and treated with the acid chloride (8 mg, 0.09 mmol). The reaction was stirred 15 min, after which time the mixture was partitioned between DCM (10 mL) and H2O (20 mL). The org layer was dried (Na2SO4) and concentrated to provide the product as a brown solid. [60 mg, 85%]
[Patent Reference: WO2014149164, page 211, ![]() (23.7 MB)]
(23.7 MB)]
Example 7
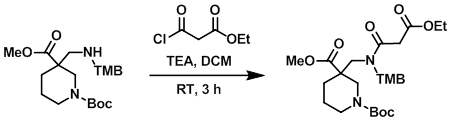
To a solution of the amine (15.0 g, 33.0 mmol) in DCM (350 mL) at 0 C was added TEA (6.7 g, 66.0 mmol) and the acid chloride (5.9 g, 40.0 mmol). The reaction was stirred at RT for 3 h, after which time the mixture was partitioned between DCM (400 mL) and H2O (200 mL). The layers were separated and the org layer was washed with sat aq NaHCO3 (100 mL), brine (100 mL), dried (Na2SO4), and concentrated in vacuo to provide the product as a thick liquid. [10.0 g, 54%]
[Patent Reference: WO2014149164, page 215, ![]() (23.7 MB)]
(23.7 MB)]
Example 8
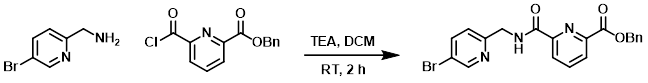
To a mixture of the amine (404 mg, 2.17 mmol) and TEA (438 mg, 4.34 mmol) in DCM (20 mL) was added the acid chloride (597 mg, 2.17 mmol) at RT. The reaction mixture was stirred at RT for 2 h. After concentration, the residue was purified by silica gel chromatography (2:1 PE/EtOAc) to provide the product as a yellow solid. [600 mg, 65%]
[Patent Reference: WO2016011390, page 126, ![]() (20.2 MB)]
(20.2 MB)]
Example 9
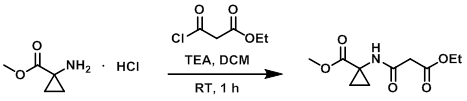
To a solution of the amine (2.0 g, 12.1 mmol) in DCM (50 mL) was added TEA (5.0 mL, 36.3 mmol) and the acid chloride (1.5 mL, 12.1 mmol). The reaction mixture was stirred at RT for 1 h. Upon completion, the mixture was quenched with sat aq NaHCO3, extracted with EtOAc, dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (30% acetone/hexane) to provide the product as a light yellow liquid. [1.3 g, 44%]
[Patent Reference: WO2014149164, page 235, ![]() (23.7 MB)]
(23.7 MB)]
Example 10
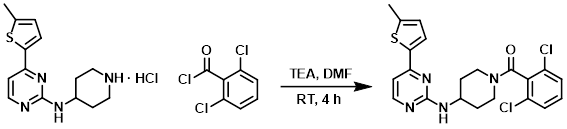
To a solution of the amine (274 mg, 1 mmol), TEA (0.69 mL, 5 mmol), and DMF (5 mL) was added the acid chloride (0.21 mL, 1.5 mmol). The reaction mixture was stirred at RT for 4 h. Upon completion EtOAc (100 mL) was added. The resulting mixture was washed with 5% LiCl (3 x 50 mL), dried (Na2SO4), concentrated, and purified by HPLC to provide the product (tan solid) as an acetate salt. [39%]
[Patent Reference: WO2007089768, page 224, ![]() (20.6 MB)]
(20.6 MB)]
