Substitution (Triflate)
(N-Heteroaryls)
Examples:
Example 1
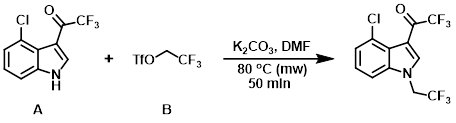
A mixture of the indole (A) (0.500 g, 2.0 mmol), the triflate (B) (0.57 mL, 4.0 mmol), and K2CO3 (0.828 g, 6.0 mmol) in DMF (2 mL) was stirred at 80 C for 50 min in a microwave reactor. The mixture was cooled to RT and partitioned between H2O (30 mL) and EtOAc (80 mL). The org layer was separated, dried (Na2SO4), and concentrated to give the product as a green solid. [0.600 g, 90%]
[Patent Reference: WO2016100281, page 94, ![]() (10.3 MB)]
(10.3 MB)]
Example 2

The pyrazole (A) (0.500 g, 3.96 mmol) was dissolved in dry ACN (30 mL), then the triflate (B) (0.633 mL, 4.76 mmol) was added, followed by Cs2CO3 (1.94 g, 5.95 mmol). The reaction mixture was stirred at 60 C for 2 h, after which time it was cooled to RT and diluted with EtOAc. Celite was added and the solvent was removed in vacuo. The residue was purified by flash chromatography (solid loading on celite, 0-60% EtOAc/hexane) to provide two products (C, 0.271 g, 36%, eluted at ~25% EtOAc) (D, 0.398 g, 53%, eluted at ~45% EtOAc). [Total yield: 0.669 g, 89%]
[Patent Reference: WO2016010950, page 162, ![]() (18.8 MB)]
(18.8 MB)]