Substitution (Br)
(Aromatic Thiols)
Examples:
Example 1
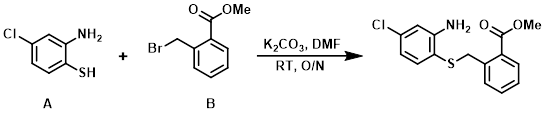
A mixture of the thiophenol (A) (3.5 g, 21.9 mmol), the alkyl bromide (B) (5.0 g, 21.9 mmol), and K2CO3 (15 g, 109.7 mmol) in DMF (50 mL) was stirred at RT overnight. The reaction mixture was poured into H2O (50 mL) and extracted with EtOAc (2 x 50 mL). The org layer was washed with brine, dried (Na2SO4), and concentrated in vacuo. The crude material was purified by silica gel column chromatography (0-30% EtOAc/hexane) to provide the product as a solid. [5.25 g, 78%]
[Patent Reference: WO2015084869, page 33, ![]() (1.9 MB)]
(1.9 MB)]
Example 2
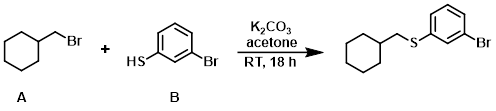
The thiophenol (B) (1.0 mL, 8.46 mmol) was added to a mixture of the alkyl bromide (A) (1.53 g, 8.61 mmol), K2CO3 (2.47 g, 17.90 mmol), and acetone. The reaction was stirred at RT for 18 h, after which time it was filtered and the filter cake was washed with acetone. The filtrate was concentrated in vacuo to provide the product as a light yellow oil. [2.37 g, 99%] [UK Pat App GB2463151A, page 118]
