Substitution (Br)
(Aliphatic Alcohols)
Examples:
Example 1
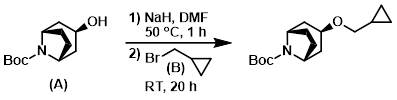
A RBF was charged with crude alcohol (A) (5.5 g) in dry DMF (25 mL) under Ar. NaH (60% in oil, 0.968 g, 24.2 mmol) was added in portions and the mixture was stirred at 50 C 1 h. The mixture was cooled to RT and (bromomethyl)cyclopropane (B) (3.252 g, 24.2 mmol) was added, followed by stirring at RT for 20 h under Ar. The reaction mixture was quenched with H2O and extracted with EtOAc. The combined organics were dried (Na2SO4), concentrated, and purified by silica gel column chromatography (1:2 EtOAc/heptane) to provide the product. [3.354 g]
[Patent Reference: WO2014152144, page 45, ![]() (4.6 MB)]
(4.6 MB)]
Example 2
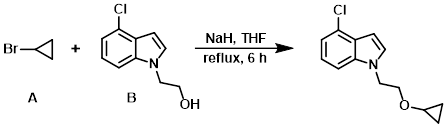
To a solution of the alcohol (B) (1.95 g, 10 mmol) in THF (13 mL) was added NaH (60% in mineral oil, 0.80 g, 20 mmol) and the alkyl bromide (A) (1.82 g, 15 mmol) at 0 C. The resulting mixture was refluxed for 6 h, then concentrated in vacuo. The residue was dissolved in EtOAc (20 mL) and washed with H2O (2 x 20 mL), brine (20 mL), dried (MgSO4), and concentrated. The resulting material was purified by silica gel column chromatography (1:5 to 1:2 EtOAc/PE) to provide the product as a yellow solid. [1.53 g, 65%]
[Patent Reference: WO2016100281, page 113, ![]() (10.3 MB)]
(10.3 MB)]