SNAr (F)
(Aromatic Alcohols)
Examples:
Example 1
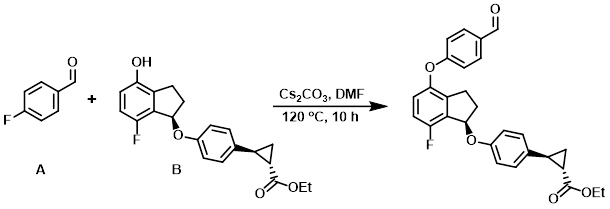
The alcohol (B) (1.20 g, 3.37 mmol), the aryl fluoride (A) (460 mg, 3.71 mmol), and Cs2CO3 (1.21 g, 3.71 mmol) were suspended in dry DMF and stirred at 120 C for 10 h. The reaction mixture was cooled to RT, diluted with H2O, and extracted with EtOAc. The org extract was dried (Na2SO4) and concentrated to provide the crude product. [1.60 g]
[Patent Reference: WO2015078802, page 62, ![]() (3.8 MB)]
(3.8 MB)]
Example 2
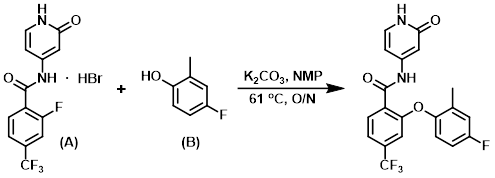
To a 50 L jacketed glass reactor fitted with an N2 inlet and a mechanical stirrer was added NMP (3.75 L). The solution was stirred, the aryl fluoride (A) (1500.2 g, 3.94 moles, 1.0 eq) was added and chased with NMP (1 L), and the jacket temperature was adjusted to 35 C. K2CO3 (325 mesh, 1631.9 g, 11.8 moles, 3.0 eq ) was then added portionwise over 10 min, during which time the reaction temp increased to 40 C. The resulting suspension was treated with a solution of the phenol (B) (546.1 g, 4.33 moles, 1.1 eq) in NMP (2.25 L) with stirring, and the addition funnel was then rinsed with NMP (0.75 L) to give an orange suspension. The jacket temperature was raised to 61 C over 30 min and the suspension was stirred overnight under N2, at which time the reaction was judged to be complete by HPLC analysis. To the reaction mixture was added 2-MeTHF (15 L) and H2O (15 L) and the mixture stirred until all solids dissolved. Stirring was stopped, the orange aq layer was drained off, and the org layer was washed with H2O (7.5 L) while stirring and a jacket temp of ~52 C. The aq wash procedure was repeated 4 times (3 x 7.5 L H2O washes, 1 x 4.5 L H2O wash). The resulting org slurry was stirred at a jacket temp of 50.8 C, and isopropyl acetate (6 L) was added. The jacket temp was ramped down to 20 C over 30 min, and the slurry was stirred overnight before collecting the precipitated solids by filtration. The collected solids were returned to the reactor, slurried in isopropyl acetate with stirring for about 2 h, then filtered, rinsed with isopropyl acetate (1.5 L), and dried in vacuo at 65 C to provide the product as an off-white solid. [1253.1 g, 78%]
[Patent Reference: WO2015089361, page 77, ![]() (5.2 MB)]
(5.2 MB)]
Example 3

To a solution of the alcohol (A) (30 mg, 0.15 mmol) in NMP (3 mL) was added the aryl fluoride (B) (42 mg, 0.44 mmol) and K2CO3 (60 mg, 0.44 mmol) at RT. The reaction mixture was heated in a microwave reactor at 160 C for 1 h. The mixture was filtered and the crude material was purified by reverse phase chromatography to provide the product as a light brown solid. [40 mg, 69%]
[Patent Reference: WO2016010950, page 256, ![]() (18.8 MB)]
(18.8 MB)]
Example 4
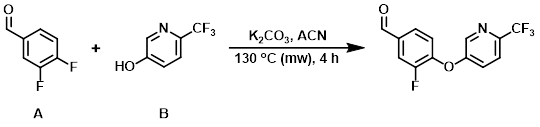
To a solution of the aryl fluoride (A) (500 mg, 3.52 mmol) and the alcohol (B) (574 mg, 3.52 mmol) in ACN (10 mL) was added K2CO3 (729 mg, 5.28 mmol). The reaction mixture was sealed and heated in a microwave reactor (Biotage Initiator) at 130 C for 4 h. After cooling, the mixture was filtered and concentrated in vacuo to provide the product as a brown solid. [903 mg, 90%]
[Patent Reference: WO2016011930, page 131, ![]() (15.7 MB)]
(15.7 MB)]
