PMB Protection
(PMB-Cl)
Examples:
Example 1
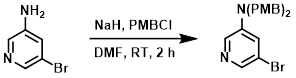
To a solution of the SM (1 g, 5.81 mmol) in DMF (10 mL) at 0 C was added NaH (512 mg, 12.8 mmol). PMBCl (2.09 g, 13.37 mmol) was added, and the mixture was stirred at RT for 2 h. The mixture was diluted with H2O (50 mL) and filtered. The resulting material was purified by silica gel chromatography (10:1 PE/EtOAc) to provide the product as a yellow oil. [1.4 g, 58%]
[Patent Reference: WO2016011390, page 84, ![]() (20.2 MB)]
(20.2 MB)]
Example 2
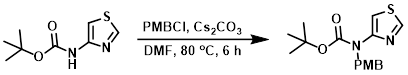
To a solution of the SM (13.0 g, 64.9 mmol) in DMF (150 mL) was added Cs2CO3 (42.3 g, 130 mmol) and PMBCl (12.1 g, 78.0 mmol). The reaction mixture was stirred at 80 C for 6 h. The mixture was allowed to cool to RT and H2O (500 mL) was added. The aq layer was extracted with ether (2 x 500 mL). The combined organics were dried (Na2SO4) and concentrated. The resulting crude material was purified by silica gel column chromatography (0-20% EtOAc/hexane) to provide the product as an off-white solid. [15.0 g, 80%]
[Patent Reference: WO2014201173, page 231, ![]() (19.7 MB)]
(19.7 MB)]
Example 3
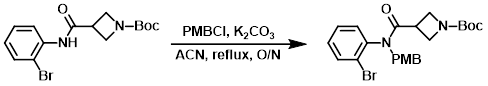
A mixture of the SM (307 g, 0.864 mol), PMBCl (203 g, 1.296 mol), and K2CO3 (358 g, 2.593 mol) in ACN (3.0 L) was refluxed overnight. The mixture was filtered and the solids were washed with ACN (1.0 L). The filtrate was concentrated in vacuo and the crude product was triturated in PE/EtOAc (30:1) to provide the product. [380 g, 90%]
[Patent Reference: WO2015158653, page 27, ![]() (2.9 MB)]
(2.9 MB)]
