Ketone to Difluoro
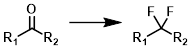
Common Conditions:
DAST
DAST is a nucleophilic fluorinating reagent. DAST is not as reactive towards ketones as it is with alcohols but conditions are still typically mild (0 C to RT). DAST can be unstable if heated (possible detonation at >90 C). The solvent of choice is usually DCM.[1][2][3]

Deoxo-Fluor
Deoxo-Fluor is a nucleophilic fluorinating reagent with similar and, in some cases, superior reactivity to DAST. Deoxo-Fluor is more thermally stable than DAST.[3]
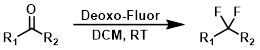
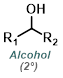
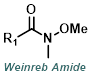
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
 |
|||
 |
 |
||
 |
References:
1) Smith, M. B.; March's Advanced Organic Chemistry
2) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups
3) Singh, R. P.; Shreeve, J. M.; Synthesis 2002, No. 17, 2561-2578
