Curtius Rearrangement
(Boc Protected Pdt)
Examples:
Example 1

DPPA (642 mg, 2.34 mmol) and TEA (236 mg, 2.34 mmol) were added to a solution of the SM (300 mg, 2.34 mmol) in t-BuOH (10 mL) at 50 C. The reaction mixture was stirred at 90 C for 16 h. After concentration, the residue was purified by silica gel column chromatography (1:4 PE/EtOAc) to provide the product as a yellow solid. [412 mg, 66%]
[Patent Reference: WO2015089337, page 151, ![]() (17.5 MB)]
(17.5 MB)]
Example 2
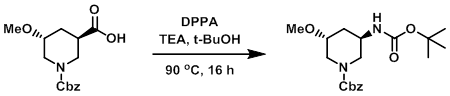
To a mixture of the SM (140 mg) dissolved in t-BuOH (2.5 mL, 0.2M) was added TEA (93 uL, 0.67 mmol). The mixture was then treated with DPPA (0.124 mL, 0.572 mmol) and stirred at 90 C for 16 h. The mixture was diluted with H2O and EtOAc. The layers were separated and the aq layer was further extracted with EtOAc (5 x 20 mL). The combined organics were dried (Na2SO4) and concentrated. The resulting material was purified by chromatography (0-100% EtOAc/heptanes) to provide the product as a clear oil. [100 mg, 58%]
[Patent Reference: WO2010016005, page 235, ![]() (11.3 MB)]
(11.3 MB)]
Example 3

To a mixture of the SM (1.25 g, 3.84 mmol), TEA (0.6 mL, 4.61 mmol), t-BuOH (8 mL), and dioxane (20 mL) was added DPPA (1.27 g, 4.61 mmol) at RT. The reaction mixture was stirred at reflux for 3 h, then concentrated in vacuo. The residue was purified by silica gel column chromatography (6:1 PE/EtOAc) to provide the product as a brown solid. [793 mg, 50%]
[Patent Reference: WO2016011390, page 143, ![]() (20.2 MB)]
(20.2 MB)]
Example 4
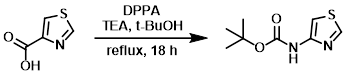
To a suspension of the SM (19.6 g, 151 mmol) in t-BuOH (400 mL) was added TEA (24.8 mL, 181 mmol) and DPPA (41.7 mL, 182 mmol). The reaction mixture was stirred at reflux for 18 h. The mixture was cooled to RT and concentrated in vacuo. The residue was diluted with EtOAc (500 mL), washed with H2O (500 mL), sat aq NaHCO3 (500 mL), dried (Na2SO4), and concentrated. The resulting crude material was purified by silica gel column chromatography (0-20% EtOAc/hexanes) to provide the product as an off-white solid. [20.0 g, 66.6%]
[Patent Reference: WO2014201173, page 231, ![]() (19.7 MB)]
(19.7 MB)]
